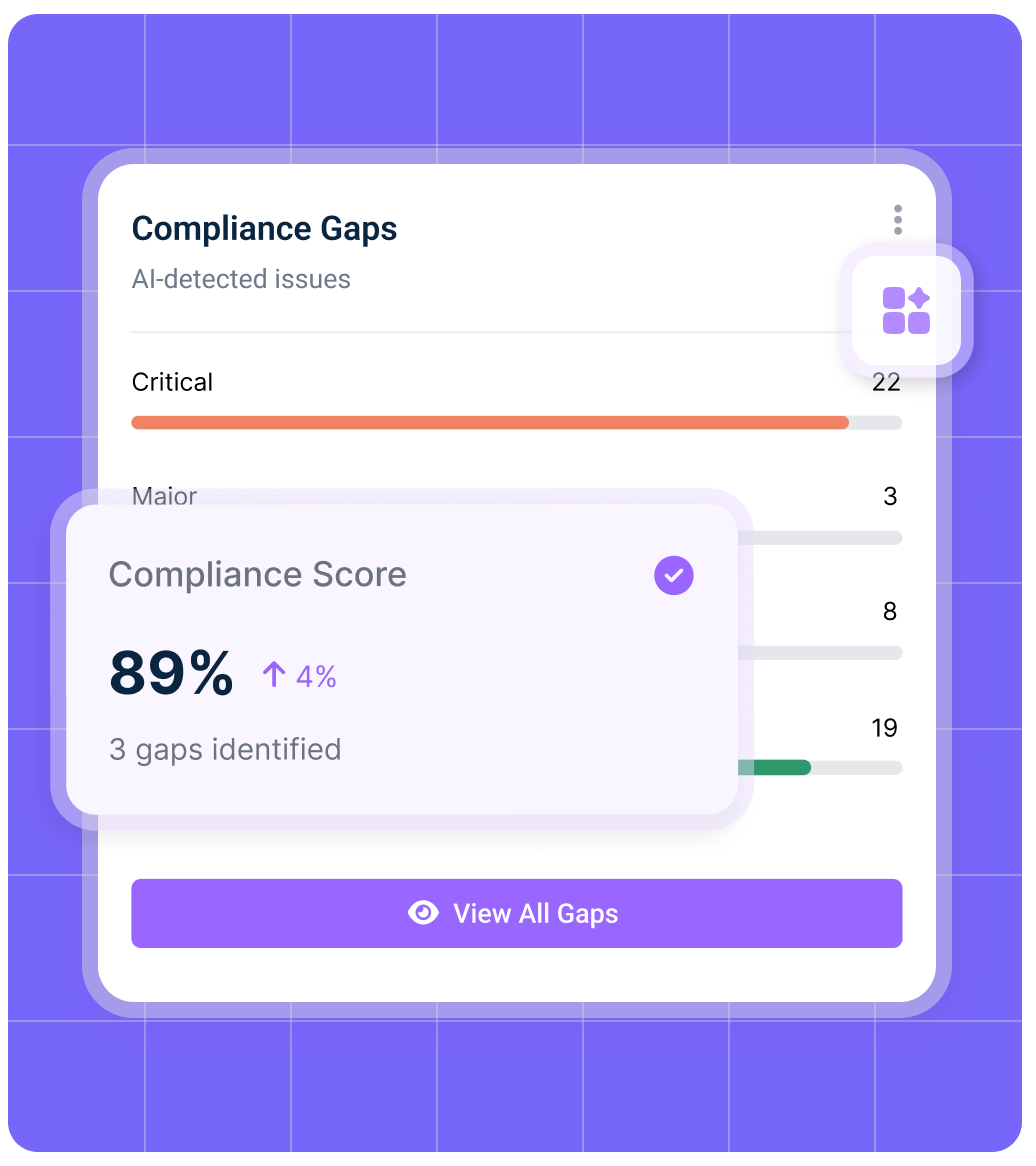
IONI’s pharma regulatory software tracks global regulators including the FDA, EMA, MHRA, PMDA, Health Canada, ICH, and WHO. Our platform is designed to support life sciences regulatory compliance, ensuring pharmaceutical, biotech, and medical device companies remain aligned with both regional and international requirements.