
Introduction: Why Regulatory Intelligence Matters More Than Ever
“In five to ten years, a lot of what we will be doing in regulatory intelligence and other parts of the pharmaceutical industry will be aided with AI.” - Ashraf, editor and regulatory expert
As far as the pharmaceutical industry expands across borders with evolving health regulations, the ability to anticipate and adapt to regulatory changes has become a competitive advantage. Companies can no longer afford to treat compliance as a reactive process.
Regulatory Intelligence in Pharma has become a critical part of how pharmaceutical companies navigate increasingly complex global compliance requirements. What used to be a quiet function is now at the forefront of strategic planning, market entry decisions, and risk management.
The volume of regulatory updates from agencies around the world - FDA, EMA, MHRA, PMDA, and more - is growing daily. New requirements, evolving expectations, and regional variations mean that pharmaceutical companies can no longer afford a passive approach.
Pharmaceutical regulatory intelligence is the engine that helps teams process, interpret, and act on this constant flow of information.
But this is just the beginning. As AI technologies continue to advance, they are starting to reshape how regulatory teams operate. Ashraf’s quote introduces a growing reality: smarter tools will increasingly take on the heavy lifting of data collection, trend analysis, and even strategic foresight. The new chapter in pharmaceutical regulatory intelligence is being written with algorithms and automation at its core.
So - what exactly is regulatory intelligence in pharma, how does it work, and why does it matter now more than ever? Let’s get into it.
What Is Regulatory Intelligence in the Pharmaceutical Industry?
Regulatory Intelligence in Pharma refers to the systematic process of gathering, analyzing, and applying regulatory information to inform business and development strategies across the pharmaceutical lifecycle. Pharmaceutical regulatory intelligence is about transforming vast amounts of fragmented data into actionable insights that help companies make informed, strategic decisions.
Under the hood, regulatory intelligence connects policy, compliance, and strategy. It plays a critical role in nearly every phase of drug development - from pre-clinical research to commercialization: by identifying evolving regulatory requirements, highlighting emerging trends, and enabling faster, better-informed decisions. This makes regulatory intelligence in the pharmaceutical industry essential for ensuring compliance and protecting revenue, reducing risks, and accelerating time to market.
The scope of RI is multifaceted:
- It includes monitoring sources such as regulatory agency websites, scientific publications, competitor activity, and industry consultations.
- It turns that information into forward-looking strategies by anticipating changes in regulations.
- It helps companies prepare for regulatory meetings, respond quickly to policy shifts, and align product strategies with evolving requirements.
The cost of non-compliance is huge. Companies without a robust RI function risk not only delays or failed approvals, but also fines, reputational damage, and lost market opportunities. That's why leading firms - especially those operating globally - treat regulatory intelligence as a core function, not a supporting one.
Moreover, in many organizations, regulatory intelligence and policy are closely connected. RI provides the data-driven insights, while policy teams use those insights to engage with regulators and industry bodies, influencing the future of the regulatory environment itself.
The Key Components of Pharmaceutical Regulatory Intelligence
A robust Regulatory Intelligence in Pharma framework depends on a combination of structured processes, specialized tools, and cross-functional coordination. It’s about tracking new regulations and understanding them in context, predicting their impact, and integrating that knowledge into strategic decision-making. For companies, this capability directly affects time to market, resource allocation, and regulatory success.
Here’s a closer, technical breakdown of the core components:
1. Regulatory Surveillance and Source Mapping
Effective pharmaceutical regulatory intelligence begins with precise and continuous surveillance of a diverse array of information sources. This includes:
- National and regional regulatory agency portals (e.g., FDA, EMA, PMDA, TGA, NMPA).
- Guidance documents, legislative proposals, and public consultations.
- Official health authority communications (e.g., warning letters, deficiency responses, Q&A updates).
- Submissions and approval histories, including approval timelines and decision summaries.
- Meeting minutes and interactions from advisory committees and sponsor reviews.
- Industry intelligence (competitor filings, press releases, market authorization insights).
- Scientific literature, clinical trial registries, and real-world evidence sources.
2. Signal Detection and Data Prioritization
Once gathered, raw regulatory data is triaged based on:
- Relevance to therapeutic areas or target molecules.
- Impact on ongoing or planned clinical trials.
- Potential for submission disruption.
- Relation to strategic partnerships or licensing terms.
Using AI-assisted platforms or semi-automated tagging systems, RI teams classify updates based on urgency, jurisdiction, and regulatory type (e.g., labeling, pharmacovigilance, manufacturing, quality, etc.).
For example, a change in ICH M7 guidance would be tagged high-priority for a company with oncology assets under development.
3. Analysis and Strategic Insight Generation
Regulatory intelligence isn’t useful unless it’s interpreted in context. Technical teams apply:
- Comparative analysis between countries or regions (e.g., FDA vs. EMA trial design requirements).
- Trend mapping to detect shifts in agency focus (e.g., digital endpoints, real-world data acceptance).
- Regulatory timelines benchmarking, such as median time to approval or approval success rates per indication.
- Gap analysis vs. current regulatory strategies and submission readiness.
4. Lifecycle Integration and Regulatory Strategy Alignment
RI must align across every phase of the drug lifecycle:
- Pre-IND/IMPD: Identify regulatory precedents, adaptive trial models, orphan/fast-track opportunities.
- Clinical development: Inform protocol design, site selection, and patient population strategies.
- Registration phase: Optimize dossier structure (eCTD granularity, Module 2 harmonization), prepare for region-specific queries.
- Post-actions: Monitor evolving safety regulations (e.g., Risk Evaluation and Mitigation Strategies - REMS, Risk Management Plans - RMPs), pharmacovigilance system updates, and variations.
5. Stakeholder Collaboration and Internal Dissemination
Clear and timely communication of regulatory intelligence is essential. Techniques include:
- Customized alerts by product, geography, or functional role.
- Internal training sessions and workshops (e.g., quarterly compliance briefings).
- Automated knowledge bases and internal wikis for storing interpretations and regulatory Q&A.
- Cross-functional RI steering committees, including clinical, legal, CMC, and market access teams.

Overall, Regulatory Intelligence in Pharma is a dynamic, interconnected discipline built on precision monitoring, sophisticated data interpretation, strategic alignment, and enterprise-wide integration.
Benefits of Regulatory Intelligence in Pharma
Having explored the structural elements of pharmaceutical regulatory intelligence, it's now important to understand the measurable value this function delivers.
Far from being just a compliance safety net, Regulatory Intelligence in Pharma is an enterprise-wide performance driver. These benefits materialize in accelerated processes, strategic foresight, and stronger regulatory positioning.
Here’s a closer look at the detailed benefits, now backed by the insights and KPIs:
1. Increased Compliance and Audit Readiness
A strong RI function directly contributes to:
- Reduced the number of warning letters and critical observations during inspections.
- Fewer “List of Questions” (LoQs) from authorities during assessments.
- Higher inspection success rates across clinical, manufacturing, and post-marketing activities.
Mature RI teams actively benchmark the speed at which regulatory changes are detected and communicated internally - ensuring faster adaptation than external sources or competitors.
2. Operational Efficiency Gains
Using well-managed RI tools (dashboards, automated newsletters, searchable databases), companies can:
- Deliver timely alerts and impact assessments tailored to specific product teams.
- Track usage metrics like page views, document downloads, and alert subscriptions to measure internal engagement.
- Reduce reliance on ad hoc regulatory requests by providing self-service RI access across departments.
This leads to improved productivity and reduced time spent manually checking health authority websites - aligning teams around unified, up-to-date insights.
3. Faster Time to Market
RI contributes to faster market entry through:
- Fewer delays in submission preparation, thanks to clarity on formatting, expectations, and recent reviewer decisions.
- Early alignment with regulatory pathways that reduce friction (e.g., accelerated assessments, rolling reviews).
- Decreased back-and-forth with regulators through more accurate, region-specific dossier preparation.
Internal KPIs tracked by RI functions include:
- Time saved in preparing application dossiers
- Reduction in regulator questions post-submission
- Lag time between submissions in mature vs. emerging markets
4. Lower Cost of Development
Key cost savings are realized through:
- Optimized protocol design (reducing amendments) based on regulatory trends.
- Greater use of real-world evidence to meet endpoint requirements with smaller populations.
- Efficient recruitment strategies aligned with region-specific inclusion/exclusion guidance.
Leveraging RI to anticipate shifting regulator expectations (e.g., decentralization, digital endpoints) allows teams to de-risk trials before first patient in (FPI), reducing costly post-launch modifications.
5. Strategic Policy Engagement and Influence
Beyond internal operations, RI enables companies to actively shape the regulatory environment:
- Number of comments submitted on draft guidance.
- Acceptance rate of those comments.
- Membership in trade association working groups.
- Feedback received from agencies on policy input.
By contributing to regulatory conversations early, companies can position themselves as proactive collaborators, gaining trust and influence with key health authorities.
6. Establishing Leadership and Reputation
RI activities strengthen a company’s external profile through:
- Conference presentations, peer-reviewed publications, and advisory participation.
- Invitations to join consortia or expert groups.
- Increased citations and mentions of company insights or best practices.
For emerging companies or those expanding into new geographies, RI visibility is crucial for brand-building and credibility.
7. Empowering Informed Strategic Decision-Making
Perhaps the most impactful benefit is that RI supports better long-term planning through:
- Roadmaps of emerging trends to guide investment and development focus.
- Data-driven advice to internal stakeholders for risk management.
- Cross-functional alignment (e.g., with market access, legal, clinical) to avoid siloed planning.
RI functions are increasingly being recognized as internal consultants, providing foresight and context that drive business-critical decisions.
Measuring Value Through KPIs
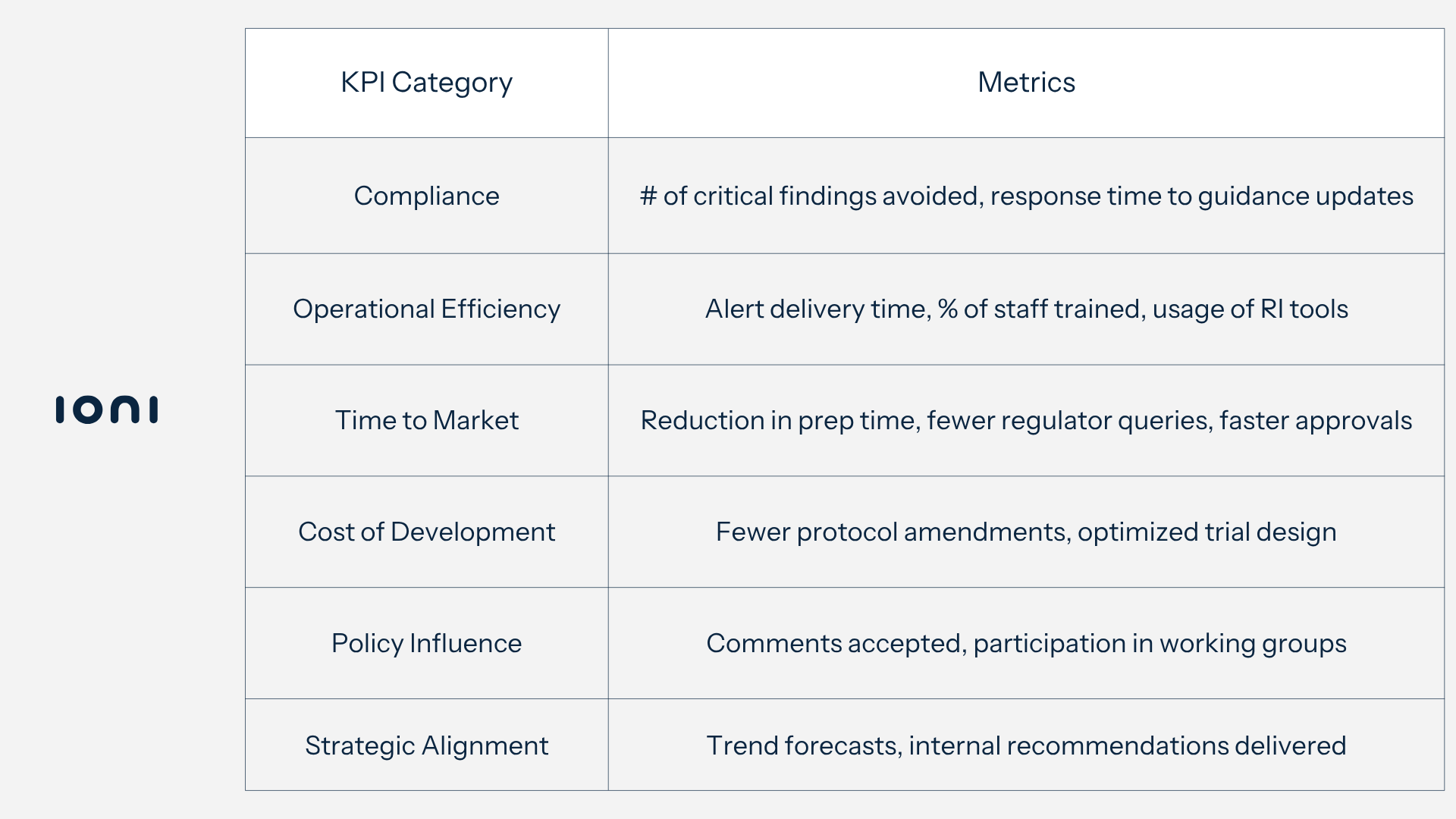
Common Pitfalls and How to Avoid Them
Despite the growing recognition of Regulatory Intelligence in Pharma as a critical function, many organizations still struggle to operationalize it effectively. While the benefits of pharmaceutical regulatory intelligence are clear: faster submissions, improved compliance, better strategy - those gains are often held back by internal barriers, outdated processes, or a lack of visibility.
To unlock the full value of regulatory intelligence in the pharmaceutical industry, it’s essential to identify the recurring obstacles - and build structured approaches to overcome them.
1. Treating RI as Invisible
One of the most pervasive issues is that RI teams operate in the background with limited visibility. They are often consulted only after a compliance issue arises.
Solution: Create regular engagement opportunities - such as presentations at cross-functional meetings, internal newsletters, and training sessions - to increase awareness of how RI supports broader company goals. Embedding RI into the early stages of development and regulatory planning helps prevent downstream issues.
2. Lack of Performance Metrics
When companies don’t track the output or impact of RI activities, leadership may undervalue the function as non-strategic.
Solution: Define and track KPIs, such as:
- Number of alerts processed and acted upon
- Speed of communication vs. competitors or third-party databases
- Stakeholder feedback on usefulness
- Regulatory timelines influenced by RI insights
3. Fragmented Communication and Siloed Functions
Without centralized systems, regulatory intelligence often stays isolated within one department. Other teams may not know who to ask, what’s available, or how to use the insights.
Solution: Use collaborative platforms or RI hubs where stakeholders can access curated updates, regulatory comparisons, and historical precedents. Cross-train functions like clinical, CMC, and market access to understand and utilize RI deliverables more effectively.
4. Manual Workflows and Lack of Automation
Trying to track regulatory changes using manual methods - like spreadsheets or inbox alerts - is unsustainable and error-prone, especially in a global environment.
Solution: Leverage robust tools such as IONI or comparable solutions to automate:
- Regulatory surveillance across jurisdictions
- Delivery of custom alerts/newsletters
- Country-to-country comparison tables
- Integration of updates into existing workflows
This frees up valuable time for strategic tasks like analysis and insight generation.
5. Information Overload and Lack of Prioritization
Even with a strong toolset, some teams drown in regulatory information without a clear process to filter, classify, and act.
Solution: Implement a triage and tagging process to assign relevance scores or labels based on therapeutic area, region, product phase, or urgency. Include impact assessments alongside alerts to help end-users focus on what matters most.
6. Decentralized Data and Version Conflicts
Without a single source of truth, teams risk working off inconsistent, outdated, or conflicting interpretations of regulatory expectations.
Solution: Establish a centralized RI repository with version control, governance policies, and defined ownership. Ensure it’s integrated with your regulatory submission and document management systems.
7. Limited External Engagement and Benchmarking
Companies that keep RI functions isolated from external dialogues miss opportunities to shape policy, anticipate changes, or benchmark practices.
Solution: Encourage RI participation in regulatory working groups, industry consortia, and agency feedback forums. This builds influence, sharpens insight quality, and raises the company’s profile as a regulatory thought leader.
8. Unrealistic Expectations for Small Teams
In smaller organizations or emerging biotech, RI is often seen as a “nice-to-have” rather than a critical function, resulting in underfunding or insufficient staffing.
Solution: Start with a lean model - such as a hybrid Regulatory Intelligence/Regulatory Affairs role or part-time external consultant - but ensure they have structured processes and tools. As complexity grows, scale gradually by formalizing the RI function and investing in automation.
Addressing these pitfalls is not just about improving operational flow - it’s about elevating Regulatory Intelligence in Pharma to a strategic level where it influences company direction, reduces risk, and drives innovation. By investing in the right infrastructure, processes, and cultural mindset, pharmaceutical organizations can build an RI capability that delivers long-term competitive advantage.
Global Trends Shaping Regulatory Intelligence in the Pharmaceutical Industry
The practice of Regulatory Intelligence in Pharma is evolving rapidly, driven not only by technological innovation but also by external pressures such as global health emergencies, geopolitical shifts, and the growing complexity of product types. These forces are reshaping how pharmaceutical regulatory intelligence is gathered, interpreted, and applied.
Below are the major global trends redefining the landscape of regulatory intelligence in the pharmaceutical industry:
1. Acceleration of Emergency Regulatory Pathways
In the wake of the COVID-19 pandemic, regulatory bodies worldwide developed and refined fast-track processes such as:
- Emergency Use Authorizations (EUAs)
- Conditional marketing authorizations
- Rolling reviews and adaptive pathways
While originally introduced for pandemic response, many of these models are now being institutionalized, creating permanent fast-track options for certain therapies, especially vaccines, oncology, and rare diseases.
Impact on RI: Regulatory intelligence teams must stay alert to evolving timelines, data expectations, and country-specific policies. Early detection of expedited pathway availability can significantly influence clinical and commercial strategy.
2. Greater Global Regulatory Divergence
Contrary to the goal of harmonization, many regions are introducing unique national requirements, often influenced by political, economic, or public health priorities. For example:
- China and Latin America are implementing more self-defined standards
- The EU and U.S. diverge in data expectations for digital health technologies
- Pharmacovigilance reporting thresholds vary significantly across markets
Impact on RI: Pharmaceutical regulatory intelligence must now go deeper than ICH alignment. Country-level comparisons, localized intelligence, and regulatory translation are increasingly necessary to avoid missed opportunities or compliance issues.
3. Growing Emphasis on Real-World Evidence (RWE) and Post-Market Data
Agencies like the FDA and EMA are actively developing frameworks for the regulatory use of RWE, including in support of label expansions and safety updates. This shift is also driving new requirements for data quality, transparency, and interoperability.
Impact on RI: Regulatory intelligence teams must monitor evolving definitions, pilot programs, and precedent-setting approvals. Understanding how each agency defines and applies RWE is now critical in building credible evidence packages.
4. Digitization and the Rise of Regulatory Technology (RegTech)
Digital tools and automation are now essential to manage the volume, velocity, and variety of regulatory data. Key developments include:
- Use of AI and NLP (Natural Language Processing) to analyze documents
- Structured content management systems for faster dossier assembly
- Real-time analytics for regulatory risk forecasting
Impact on RI: The role of regulatory intelligence is expanding from passive monitoring to active signal detection, impact modeling, and prediction. RI professionals are increasingly expected to work with data scientists and tech platforms to interpret complex datasets.
5. Increased Public and Political Scrutiny
Pharmaceutical regulation is no longer a behind-the-scenes process. Governments, advocacy groups, and the public are demanding:
- Greater transparency in approval decisions
- Faster access to critical medicines
- Stricter standards for safety and equity
Impact on RI: Teams must now track not only scientific guidance but also public policy, media sentiment, and legislative proposals. Regulatory intelligence is becoming broader and more multidisciplinary - extending into legal, ethical, and reputational domains.
6. Evolving Product Types and Scientific Frontiers
New product classes - such as cell and gene therapies, mRNA platforms, and digital therapeutics - are pushing the limits of current regulatory frameworks. In many cases, regulators are writing rules in real time.
Impact on RI: Intelligence teams must focus on precedent-tracking, monitoring how agencies handle first-in-class applications. There’s also a growing need to follow scientific advisory panels, draft guidelines, and stakeholder consultations to anticipate where regulations are heading.
The companies that adapt quickly - embedding pharmaceutical regulatory intelligence into every phase of development and decision-making - will be the ones best positioned to thrive in the years ahead.
Choosing the Right Tools and Partners for Effective Regulatory Intelligence
As the global regulatory landscape grows more complex and unpredictable, even the most well-staffed teams can’t keep up using traditional methods alone.
Teams that want to lead in pharmaceutical regulatory intelligence need more than expertise - they need scalable tools and trusted partners who can enhance visibility, reduce manual effort, and inject strategic insight into every decision.
Let’s explore what that looks like in practice.
1. Core Capabilities of Effective RI Platforms
Modern regulatory intelligence systems must go beyond basic alerting. Key capabilities should include:
- Automated monitoring of global regulatory sources (FDA, EMA, MHRA, PMDA, etc.)
- Customizable alerts and filters by product, region, or therapeutic area
- Built-in analytics to identify trends, risks, and opportunities
- Regulatory comparison tools to evaluate differences between countries or regions
- Centralized dashboards for tracking insights and team collaboration
- Searchable historical archives for precedent-based strategy development
Without these functions, teams risk missing critical updates or duplicating work across departments.
2. Integration with Regulatory and Clinical Workflows
RI tools should integrate seamlessly with systems already in use - such as:
- Regulatory Information Management platforms
- Document management systems
- Clinical trial planning and tracking tools
- Submission templates (e.g., eCTD guidance alignment)
This ensures that insights move directly into action rather than getting lost in email threads or siloed databases.
3. The Role of Specialized Partners
In addition to platforms, choosing the right service partners can greatly improve efficiency and quality. Outsourcing or co-developing RI capabilities with a specialized partner allows companies to:
- Fill internal resource gaps (especially in smaller or mid-sized teams)
- Gain access to subject matter experts across regions
- Stay ahead of region-specific regulatory shifts
- Receive curated updates and contextual analysis, not just raw data
Partners can also act as external validators, offering independent interpretation of emerging trends and draft guidance - something particularly useful when preparing for regulatory meetings or strategy reviews.
4. How IONI Supports Scalable, Strategic Regulatory Intelligence
Our solution IONI - regulatory intelligence provider known for blending deep pharmaceutical expertise with advanced data technology. IONI enables clients to:
- Automate monitoring of global regulatory developments
- Receive tailored alerts by market, product type, or therapeutic focus
- Access curated analysis - updates and explanations of their strategic impact
- Collaborate with experienced analysts to shape regulatory roadmaps
- Visualize regulatory change over time through interactive dashboards
What makes IONI especially valuable is its ability to adapt its services to the maturity and needs of each client. Whether you're building your first RI function or scaling a global operation, IONI provides flexible infrastructure and expert support.
Watch how IONI works
5. Questions to Ask When Choosing Tools or Partners
To make the right decision, companies should ask:
- Can the platform monitor the full range of regulatory bodies relevant to our portfolio?
- Does it support real-time alerts and smart filtering by role or geography?
- How customizable are the outputs (dashboards, reports, integrations)?
- Can partners provide localized intelligence - not just English-language summaries?
- Do analysts offer proactive trend insights or only retrospective data?
The goal is to build a system that delivers information and amplifies your regulatory intelligence function.
Conclusion
Regulatory Intelligence in Pharma is a core business function. As regulatory frameworks become more fragmented, faster, and digitally driven, the ability to anticipate, interpret, and act on change defines competitive advantage.
Pharmaceutical regulatory intelligence adds real value when it's integrated, measured, and used to inform strategy - not just compliance. With the right tools, structure, and mindset, regulatory intelligence in the pharmaceutical industry becomes a lever for smarter decisions, leaner operations, and stronger market positioning.
Now’s the time to treat it as such - and move with intent.
Ready to elevate your regulatory intelligence strategy? Let’s talk.
FAQ
Can startups or small biotechs benefit from regulatory intelligence, or is it only for large companies?
Absolutely. Even lean RI practices can help smaller companies identify faster pathways, avoid costly mistakes, and align early with evolving standards - key advantages when resources are tight.
What role does regulatory intelligence play in preparing for regulatory inspections?
Regulatory intelligence in the pharmaceutical industry can help teams anticipate inspection focus areas by analyzing trends in recent findings, enforcement actions, and regulator priorities - allowing better preparation and reduced risk of surprises.
How often should regulatory intelligence outputs be reviewed or updated?
Ideally, RI outputs (dashboards, alerts, reports) should be monitored in real-time or weekly, depending on risk level. Monthly trend analysis is common for strategic planning.
How does regulatory intelligence impact the lifecycle management of approved products?
It informs post-market activities by tracking labeling changes, new safety requirements, or global guideline shifts - ensuring pharmaceutical regulatory intelligence supports compliance and ongoing market viability.


.png)
.png)





