
Regulatory Intelligence 101: What It Is and Why It Matters
Why “101?” The point is that "101" is likely referring to a beginner's guide or an introduction to the pharmaceutical industry, often seen in titles such as "Pharma 101" or "Pharmaceuticals 101," which describe foundational information about drug development, regulations, and industry processes.
In the pharmaceutical industry, regulatory intelligence 101 refers to the process of collecting, analyzing, and applying information related to global regulatory frameworks, compliance requirements, and drug approval pathways. It plays a crucial role in enabling pharmaceutical companies to navigate complex regulations, bring safe and effective drugs to market, and maintain compliance throughout the drug development lifecycle.
Global health authorities such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are responsible for overseeing clinical trials, evaluating investigational new drug (IND) applications, and ensuring patient safety during drug development. Their guidance, together with international standards like the International Council for Harmonisation (ICH) Good Clinical Practice (GCP) guidelines, sets the foundation for how pharmaceutical companies conduct research, manage risks, and prepare regulatory submissions.
Regulatory intelligence is especially important in early-stage drug development, where ethical considerations and patient safety are paramount. Before a new drug reaches clinical trials, companies must gather extensive data on its manufacturing, quality, safety, and potential effects on the human body.
By systematically monitoring updates from regulatory bodies, pharmaceutical organizations can anticipate changes, reduce approval delays, and make more informed strategic decisions.
Where Regulatory Intelligence Comes From: U.S. and EU Regulations
Regulatory intelligence is embedded in formal requirements within regulatory frameworks and quality standards in both the U.S. and the EU.
United States
In the U.S., regulatory intelligence is reflected in FDA regulations and guidance:
- FDA Oversight: Pharmaceutical companies must comply with the Food, Drug, and Cosmetic Act (FD&C Act), which establishes requirements for drug safety, efficacy, labeling, and manufacturing. Staying updated on guidance documents, new drug approval pathways, and safety notifications is a regulatory intelligence activity.
- cGMP Requirements (21 CFR Parts 210–211): Manufacturers must continuously monitor current Good Manufacturing Practices (cGMP) to ensure product quality, maintain compliance with inspections, and implement corrective actions as needed.
- ICH Guidance Integration: FDA incorporates international harmonized standards, such as ICH Good Clinical Practice (GCP) and Good Laboratory Practice (GLP). Tracking changes in these standards is part of regulatory intelligence activities.
European Union
Within the EU, regulatory intelligence is embedded in regulations and standards for medical devices and pharmaceuticals:
Medical Device Regulation (MDR 2017/745) & In Vitro Diagnostic Regulation (IVDR 2017/746):
- Annex I – General Safety and Performance Requirements: Manufacturers must ensure devices meet safety and performance requirements throughout their lifecycle, requiring continuous monitoring of standards and regulatory changes.
- Article 10 – General Obligations of Manufacturers: Companies must establish, document, implement, and maintain risk management systems, which include staying informed about regulatory updates.
ISO 13485:2016 – Quality Management Systems for Medical Devices: Regulatory intelligence is embedded in several clauses:
- Clause 4.1.2(b) – General Requirements: Organisations must identify applicable regulatory requirements and incorporate them into their QMS, ensuring updates are monitored.
- Clause 7.2.1 – Determination of Requirements Related to the Product: Companies must determine and meet regulatory requirements for their devices, which necessitate regulatory intelligence activities.
- Clause 7.3.3 – Design and Development Inputs: Design inputs must include applicable regulatory requirements and standards, requiring continuous monitoring.
- Clause 8.2.1 – Feedback: Product performance data collection must include monitoring regulatory compliance to identify potential issues promptly.
Together, these U.S. and EU requirements highlight that regulatory intelligence is a critical function for maintaining compliance, anticipating changes, mitigating risks, and ensuring patient safety across the product lifecycle.
Why Regulatory Intelligence Is Essential in the Pharmaceutical Industry
The pharmaceutical sector operates under intense regulatory scrutiny. Developing a new drug involves multiple stages of testing, clinical trials, and safety assessments - all of which must comply with evolving global standards.
Here’s where regulatory intelligence 101 becomes indispensable: it provides the insights needed to manage compliance, avoid costly mistakes, and bring therapies to market faster.
Pharma companies face unique challenges:
- Complex approval processes - Regulatory submissions like INDs in the U.S. and CTAs in Europe require extensive documentation, from chemistry and manufacturing data to clinical safety reports.
- Constantly evolving regulations - FDA, EMA, and ICH guidelines are frequently updated to address new therapies and technologies, making real-time monitoring essential.
- Patient safety requirements - Global authorities enforce Good Clinical Practices (GCPs) to ensure ethical testing and minimize risks during clinical trials.
- Global market pressures - Companies developing drugs for multiple regions must comply with different local regulations, timelines, and reporting obligations.
Without an effective regulatory intelligence process, delays are inevitable - submissions get rejected, trials face holds, and approvals stall. On the other hand, companies that leverage regulatory intelligence gain a competitive edge by:
- Anticipating regulatory changes early
- Designing clinical trials aligned with global standards
- Reducing the risk of non-compliance penalties
- Accelerating time-to-market for life-saving drugs
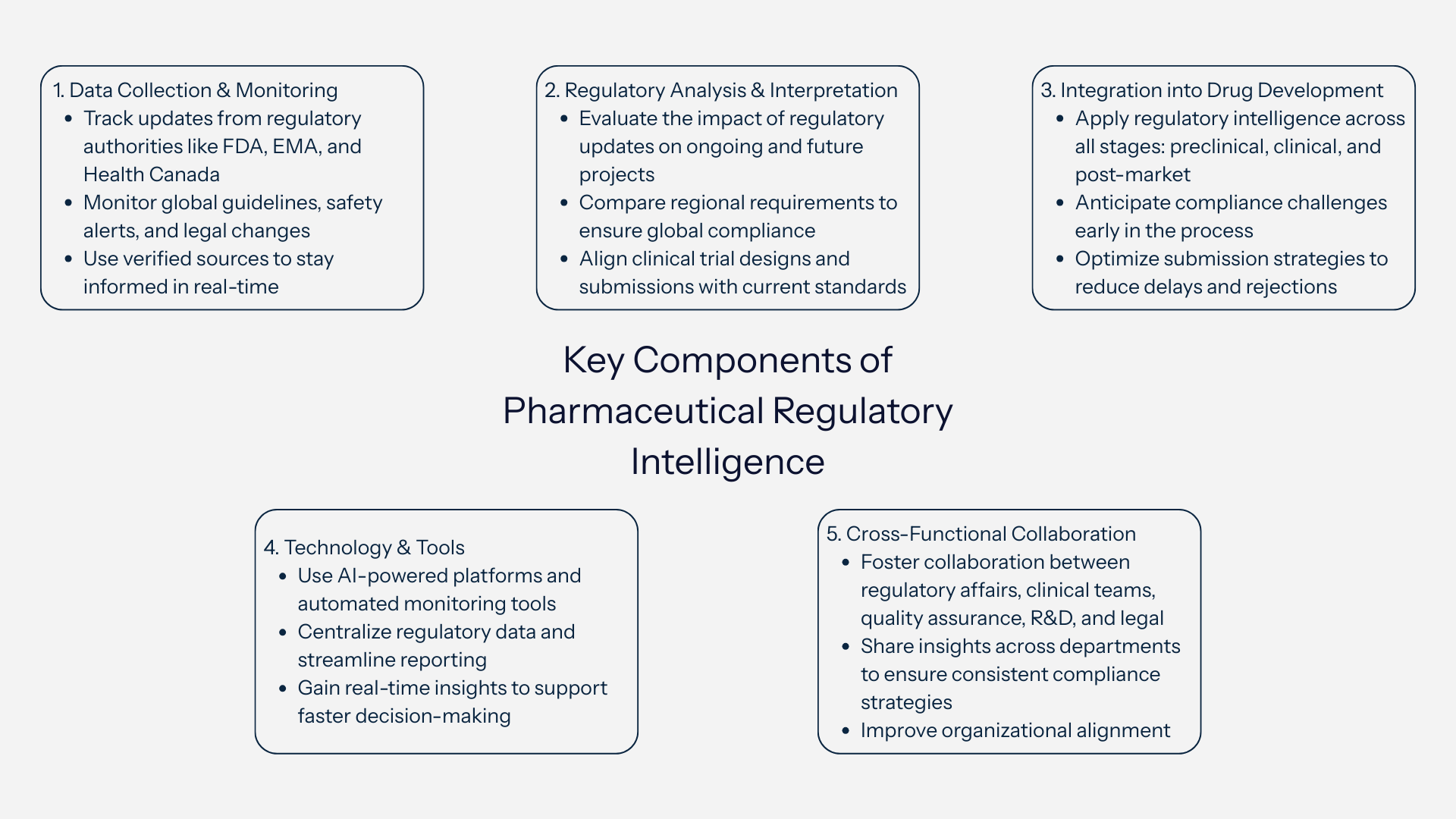
Key Components of Pharmaceutical Regulatory Intelligence
In the pharmaceutical industry, regulatory intelligence 101 involves a structured approach to gathering, analyzing, and applying regulatory data to guide strategic decisions.
To manage compliance effectively and accelerate drug development, organizations focus on several key components:
1. Data Collection and Monitoring
Pharmaceutical regulatory intelligence starts with identifying reliable data sources. These include global regulatory authorities like the U.S. FDA, European Medicines Agency (EMA), and Health Canada, as well as scientific publications, industry reports, and legal updates. Continuous monitoring of regulatory guidelines, safety alerts, and submission requirements helps companies stay ahead of evolving expectations.
Regulatory changes can occur frequently. For instance, the FDA adds new guidance documents regularly, with recent additions in July 2025. Similarly, the EMA updates its European Union Reference Dates (EURD) list monthly. Health Canada also revises its guidance documents periodically; for example, updates to the Quality (Chemistry and Manufacturing) Guidance were released in December 2024.
2. Regulatory Analysis and Interpretation
Collecting data is only the first step - the critical part is interpreting what the information means for the company’s drug development strategy. Regulatory teams evaluate updates, compare international requirements, and assess potential impacts on clinical trials, manufacturing, labeling, and marketing approvals.
3. Integration into the Drug Development Lifecycle
Regulatory intelligence plays a strategic role across all stages of drug development - from preclinical research to clinical trials and commercialization. By integrating intelligence early, teams can anticipate regulatory expectations, design compliant trials, and prepare submissions aligned with global standards, reducing the risk of delays or rejections.
4. Leveraging Technology and Tools
Modern regulatory intelligence relies on AI-powered platforms and automated monitoring tools to track changes in regulations worldwide. These tools centralize updates, flag critical changes, and provide real-time insights, making it easier for pharma teams to make informed decisions quickly.
5. Cross-Functional Collaboration
Effective regulatory intelligence requires close collaboration between regulatory affairs, clinical operations, quality assurance, legal teams, and R&D departments. Sharing insights across teams ensures that all stakeholders align on compliance strategies and respond consistently to evolving requirements.
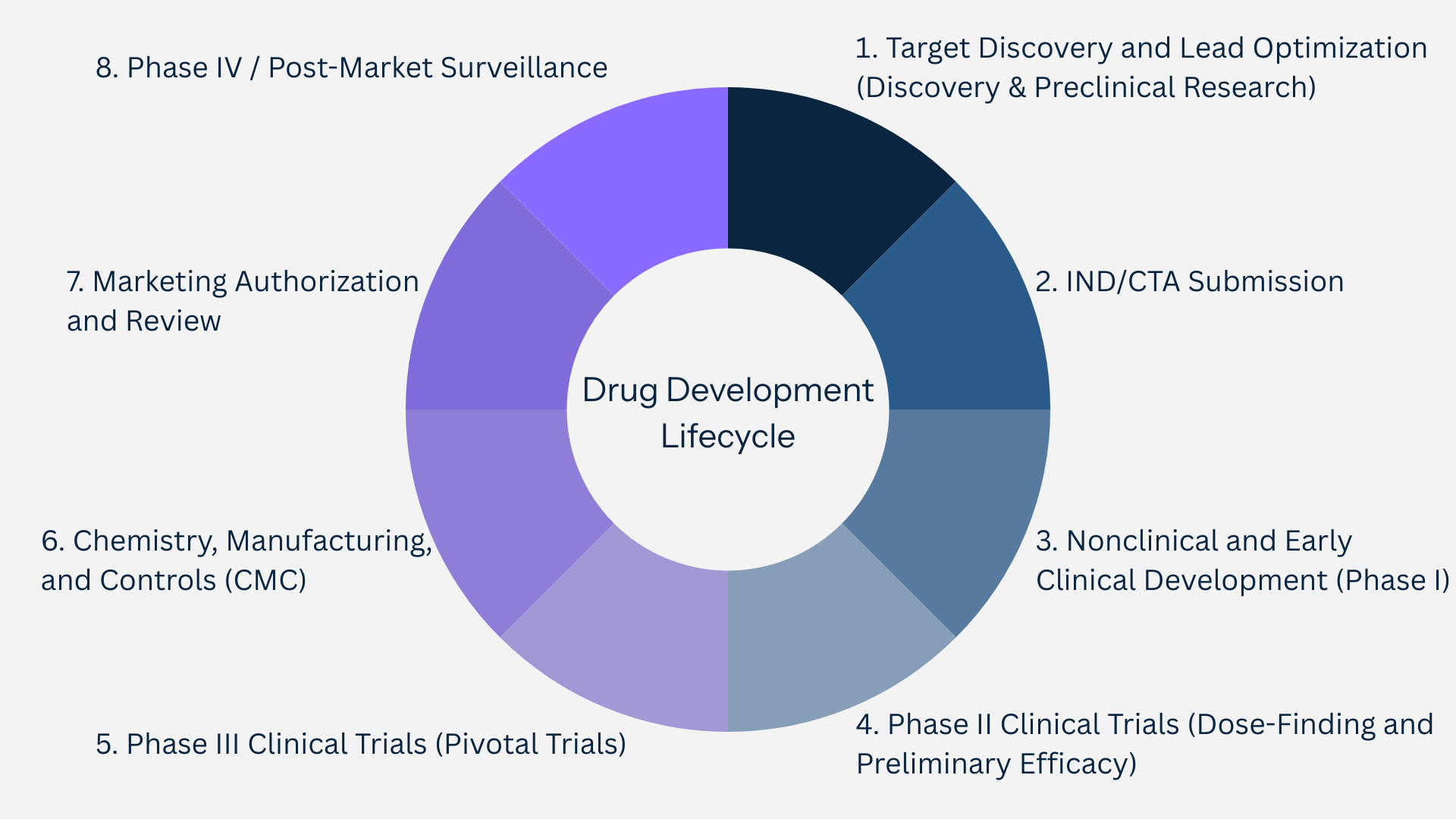
How Regulatory Intelligence Fits Into the Drug Development Lifecycle
Having reviewed the key components of pharmaceutical regulatory intelligence, it is important to see how these insights operate across the entire drug development journey. At its core, regulatory intelligence 101 ensures that every stage - from discovery to post-market surveillance - is aligned with global regulatory expectations, minimizing risk and accelerating patient access. Expedited programs such as Fast Track, Breakthrough Therapy, Accelerated Approval, and Priority Review are central to this process.
1. Target Discovery and Lead Optimization (Discovery & Preclinical Research)
At this stage, regulatory intelligence helps organizations evaluate precedent, assess therapeutic potential, and identify whether a therapy may qualify for expedited pathways.
Fast Track: Designed to speed the development and review of drugs treating serious conditions and unmet medical needs. A therapy may qualify if it offers superior effectiveness, avoids severe side effects, improves diagnosis, reduces significant toxicity, or addresses emerging public health needs. Conditions such as cancer, AIDS, Alzheimer’s, or epilepsy are typical candidates. Benefits include frequent FDA meetings, written communications, rolling review of applications, and eligibility for Accelerated Approval or Priority Review. A Fast Track request can be submitted anytime after an IND application, with FDA responding within 60 days.
2. IND/CTA Submission
Before clinical trials begin, companies submit an Investigational New Drug (IND) application to the FDA (or a Clinical Trial Application/CTA to other regulators) to demonstrate safety and justify human testing. Regulatory intelligence ensures that submission packages are complete, compliant, and strategically positioned to enable discussions about expedited programs.
3. Nonclinical and Early Clinical Development (Phase I)
As drugs move into toxicology studies and first-in-human trials, regulatory intelligence informs trial design, endpoints, and safety monitoring.
Breakthrough Therapy: Applies to drugs treating serious conditions where preliminary clinical evidence suggests substantial improvement over existing therapies on clinically significant endpoints. Unlike Fast Track, which may rely on early or nonclinical evidence, Breakthrough Therapy requires clinical data, such as improvement in irreversible morbidity, mortality, or validated surrogate endpoints. Benefits include all Fast Track features, plus intensive FDA guidance from Phase I onward and involvement of senior FDA leadership. Requests are most effective by the end of Phase II to shape later development.
4. Phase II Clinical Trials (Dose-Finding and Preliminary Efficacy)
Regulatory intelligence guides dose selection, trial design, and identification of additional expedited opportunities. It ensures endpoints are aligned with regulatory expectations and can support accelerated pathways.
5. Phase III Clinical Trials (Pivotal Trials)
During Phase II and III, regulatory intelligence helps determine if Accelerated Approval is feasible.
Accelerated Approval: First instituted in 1992 and expanded by the 2012 FDASIA Act, this pathway allows approval of drugs for serious conditions based on surrogate or intermediate clinical endpoints that are reasonably likely to predict true clinical benefit.
- Surrogate endpoints: Markers such as lab results, imaging, or physical signs thought to predict clinical benefit but not direct measures of survival or quality of life.
- Intermediate clinical endpoints: Measures of therapeutic effect (e.g., effects on irreversible morbidity and mortality) considered reasonably likely to predict benefit.
This approach allows earlier approval, but confirmatory trials are required. Regulatory intelligence ensures trials are well-designed, endpoints justified, and post-marketing commitments robust. If later studies fail to confirm benefit, the FDA may withdraw approval or modify labeling.
6. Chemistry, Manufacturing, and Controls (CMC)
Regulatory intelligence ensures that manufacturing processes, formulation, and quality controls meet global standards, avoiding delays in review and approval.
7. Marketing Authorization and Review
At the submission stage, regulatory intelligence ensures applications are strategically positioned for Priority Review or other designations.
Priority Review: Established in 1992 under PDUFA, this pathway reduces the FDA’s review goal from 10 months (Standard Review) to 6 months. Priority Review is granted when a therapy, if approved, would represent significant improvements in the treatment, diagnosis, or prevention of serious conditions. Examples include:
- Evidence of increased effectiveness.
- Elimination or substantial reduction of treatment-limiting adverse reactions.
- Improved patient compliance is expected to enhance outcomes.
Evidence of safety and efficacy in a new subpopulation.
FDA decides review designation for every NDA/BLA, but applicants can request it. FDA responds within 60 days. Priority Review does not lower approval standards - it accelerates the timeline only.
8. Phase IV / Post-Market Surveillance
Regulatory intelligence continues beyond approval to ensure compliance and lifecycle management.
- Fast Track & Breakthrough Therapy: Ongoing communication obligations, additional safety monitoring, and labeling updates may be required.
- Accelerated Approval: Sponsors must conduct confirmatory trials to validate clinical benefit. Failure to do so can result in withdrawal of approval.
- Priority Review: Drugs approved under this designation are monitored with the same rigor as standard reviews, but regulatory intelligence ensures ongoing compliance as requirements evolve.
Who Uses Regulatory Intelligence - and How?
How Regulatory Intelligence Fits Into the Drug Development Lifecycle naturally leads to the next question: who actually uses this information, and in what ways? Understanding the practical applications of regulatory intelligence is crucial for grasping its value.
In essence, regulatory intelligence 101 is about leveraging insights to make informed decisions throughout drug development. Various stakeholders rely on regulatory intelligence to optimize programs, assess feasibility, prepare for meetings, and even guide education and training efforts within the company. Let’s explore who uses regulatory intelligence, and how.
1. Chief Compliance Officer & Compliance Committee
Duties:
- Define overall compliance policies and frameworks
- Approve governance structures and risk management strategies
- Align global regulatory strategies with corporate objectives
- Oversee enterprise-wide regulatory readiness
Software Used:
- GRC platforms (e.g., Arcnofollowher, ServiceNow GRC)
- Internal compliance dashboards
How They Use Regulatory Intelligence:
- Access real-time updates on global regulations and policy changes
- Leverage dashboards for high-level risk assessments
- Set priorities for compliance units based on emerging trends and agency alerts
- Coordinate strategies across legal, regulatory, and IT security teams
2. Regulatory Affairs, Regulatory Intelligence & Compliance Officers (Core Users)
Duties:
- Perform horizon scanning to track FDA, EMA, PMDA, and other agency updates
- Analyze and interpret regulatory guidelines for submission readiness
- Advise cross-functional teams on labeling, documentation, and GxP compliance
- Collaborate closely with Legal, IT Security, Business Intelligence, and Quality Assurance teams
Software Used:
- Internal Regulatory Intelligence databases
- FDA & EMA tracking systems
- QMS/DMS solutions (Veeva Vault, MasterControl)
- SaaS platforms like IONI and Regology
How They Use Regulatory Intelligence:
- Use dashboards and AI-driven tools (e.g., IONI) for regulatory horizon scanning
- Identify gaps between internal processes and global regulatory expectations
- Provide insights for risk assessments, submission strategies, and portfolio decisions
- Maintain an up-to-date internal repository of regulatory data for other teams
3. Legal & IT Security Teams
Duties:
- Translate regulatory updates into actionable policies and SOPs
- Ensure data privacy, cybersecurity, and compliance with evolving frameworks
- Map regulatory controls into IT and operational environments
Software Used:
- Document Management Systems like Veeva Vault, MasterControl
How They Use Regulatory Intelligence:
- Convert new regulatory requirements into internal SOPs and control policies
- Work with compliance and IT teams to embed regulations into workflows
- Use regulatory updates to strengthen data protection and cybersecurity frameworks
4. Quality Assurance, Quality Control & CAPA Managers
Duties:
- Oversee GMP, GDP, and other quality standards
- Manage product release processes and deviation investigations
- Conduct root cause analysis and implement Corrective and Preventive Actions (CAPA)
- Ensure manufacturing and distribution comply with global quality frameworks
Software Used:
- Quality Management Systems like TrackWise and MasterControl
- Laboratory Information Management Systems
- Manufacturing Execution Systems
- CAPA modules (e.g., EtQ Reliance)
How They Use Regulatory Intelligence:
- Monitor quality-related regulations and adapt processes accordingly
- Adjust product testing and release strategies based on updated standards
- Integrate regulatory data into QMS to automate quality compliance tracking
- Use alerts to prevent non-compliance before audits or inspections
5. Internal Audit Teams
Duties:
- Perform internal audits, track deviations, and review CAPA implementations
- Validate compliance across operations, manufacturing, and clinical studies
- Prepare reports for leadership and regulatory inspections
Software Used:
- AuditBoard
- Internal logging systems
- QMS-integrated audit modules
How They Use Regulatory Intelligence:
- Use RI dashboards to prepare for agency inspections
- Incorporate regulatory updates into annual audit frameworks
- Flag deviations early and align processes with current expectations

By integrating regulatory intelligence into their workflows, these teams reduce risk, increase efficiency, and make informed decisions throughout the drug development lifecycle, serving as a central hub of knowledge that links strategy, operations, and compliance.
Tools and Resources for Beginners in Regulatory Intelligence
Embarking on the journey of regulatory intelligence 101 can be complex, given the vast and ever-evolving nature of global regulations. However, several tools and resources are tailored to assist beginners in building a solid foundation and effectively managing compliance requirements.
Below is an overview of some prominent platforms:
Regulatory Intelligence Platforms
1. IONI: An AI-driven compliance platform designed to automate regulatory intelligence tasks. It offers real-time insights, alerts, and risk mapping, enabling teams to stay ahead of changing regulations. IONI's features include proactive identification of compliance gaps, seamless integration with existing systems, and enterprise-grade security.
Watch how IONI works
2. Clarivate Cortellis Regulatory Intelligence: A comprehensive platform providing access to regulatory requirements for human drugs, biologics, medical devices, and IVDs across over 110 countries and regions. It offers real-time updates, cross-country comparisons, and expert summaries to support strategic planning and compliance efforts.
3. IQVIA Tarius: A dedicated platform offering global regulatory information for human drugs, biologics, medical devices, and IVDs. Tarius provides access to health authority documents, cross-country comparisons, and summaries of regulatory parameters, aiding in the determination of optimal regulatory pathways.
4. Redica Systems: A data analytics platform built for life sciences teams to improve product quality, reduce regulatory risk, and get ahead of compliance issues. Redica Systems transforms millions of data points into meaningful insights, offering tools to monitor inspections, enforcement actions, and regulatory publications.
5. The ABIS Group: Provides industry intelligence solutions to help businesses gain and maintain a competitive edge. Their services focus on helping clients discover how to obtain and retain valuable industry insights, supporting strategic decision-making in regulatory affairs.
News Organizations
Staying abreast of evolving regulations is crucial for regulatory professionals, and subscribing to industry-specific newsletters is an effective way to achieve this. While major news outlets like the Associated Press, The Wall Street Journal, and the British Broadcasting Corporation provide general news coverage, several specialized publications offer in-depth analysis and updates pertinent to the regulatory field. Some of these subscriptions may require a fee.
Below is a curated list of prominent industry-specific newsletters:
- Fierce Pharma: Delivers breaking news and analysis about drug companies, the FDA, and the broader pharma industry, including manufacturing, marketing, and policy updates.
- ECA GxP Newsletter: A free weekly newsletter from the ECA Academy, informing subscribers about the latest developments and trends in GMP/FDA compliance topics.
- FDA Law Blog: Provides up-to-date information on current issues affecting FDA-regulated companies and legislation, offering insights from experts in the field.
- Pink Sheet: Offers keen analysis on news and regulatory trends to help professionals navigate biopharma policy and inform regulatory strategies.
- PDA Letter: Offers insights into the pharmaceutical and biotechnology industries, including regulatory news, industry trends, and policy updates.
- ISPE Pharmaceutical Engineering: Provides information on pharmaceutical engineering, including regulatory updates, industry trends, and technological advancements.
- RAPS Rapporteur: Offers insights into regulatory affairs, including updates on regulations, guidance documents, and policy developments.
By subscribing to these newsletters, regulatory professionals can stay informed about the latest developments in regulations, ensuring they remain compliant and proactive in their roles.
Common Mistakes to Avoid When Starting Out
Even with the best tools and resources, beginners in regulatory intelligence 101 often face challenges that can slow progress or lead to errors. Understanding and avoiding these common mistakes can make the learning curve smoother and more effective:
- Relying on a Single Source of Information - Many beginners focus on one regulatory database or newsletter, but this can create blind spots. Regulatory requirements vary across regions, and missing updates from other sources can lead to compliance gaps. Always cross-reference multiple platforms, including FDA, EMA, IONI, and industry-specific newsletters.
- Failing to Stay Current - Regulations are dynamic. New guidance, updated standards, or policy changes can occur at any time. Skipping regular monitoring of regulatory updates can result in outdated practices and missed compliance deadlines. Setting up alerts and scheduling periodic reviews helps maintain up-to-date knowledge.
- Ignoring Regional Differences - Regulatory intelligence is global, but each region has unique rules and expectations. Assuming that FDA requirements automatically align with EMA or Health Canada standards can cause mistakes. Beginners should always verify requirements for each market separately.
- Neglecting Documentation - Collecting regulatory information is not enough; it must be organized, documented, and easily retrievable. Poor documentation can lead to inefficiencies, repeated work, and difficulty demonstrating compliance during audits or inspections.
- Overlooking Context and Analysis - Simply collecting updates is not sufficient. Regulatory intelligence 101 requires analyzing the implications of regulatory changes on ongoing projects, risk management, and product development strategies. Failing to interpret the impact of new rules can result in inappropriate or delayed responses.
- Underestimating the Value of Training and Networking - Beginners sometimes try to go it alone, but learning from experienced colleagues, professional networks, or regulatory affairs communities can accelerate understanding and provide practical insights. Forums, workshops, and mentorship can prevent common beginner mistakes.
- Not Integrating Tools Effectively - Platforms like IONI, Clarivate Cortellis, or Redica Systems are powerful, but only if used strategically. Treating them as “set-and-forget” solutions without incorporating their insights into workflows limits their usefulness. Regularly review alerts, integrate updates into QMS, and ensure actionable steps are taken.
By being aware of these pitfalls, beginners can build a strong foundation in regulatory intelligence 101, develop robust compliance practices, and contribute more effectively to their organization’s regulatory strategy.
Getting Started: Building a Basic Regulatory Intelligence Process
Having recognized the common pitfalls that beginners encounter, the next step in mastering regulatory intelligence 101 is to establish a structured and practical approach. Building a basic regulatory intelligence process provides a foundation for systematically gathering, analyzing, and applying regulatory information. By following a clear process - such as one supported by platforms like IONI - regulatory affairs teams can avoid errors, stay organized, and ensure that regulatory updates translate into actionable insights.
1. Identifying and Setting Up Regulatory Sources
The process begins by defining where your regulatory information comes from. In IONI:
- Account-Specific Configuration: Each company can select sources based on the jurisdictions and regulations relevant to its operations. This ensures regulatory intelligence is tailored to business needs.
- Source Types:
- Automated: API integrations with official sites, such as EU legislation portals, FDA databases, or other recognized sources.
- Manual: Some sources require human input or adjustments due to complexity or format limitations.
- Unlimited Sources: Companies can integrate as many sources as needed to achieve comprehensive coverage.
- Source Management: A dedicated Sources Dashboard allows users to view the status of each source, manage updates, and monitor activity.
2. Monitoring and Initial Filtering of Regulations
Once sources are integrated, IONI continuously monitors them for updates and new regulations:
- Automated Alerts: Notifications for newly published or updated regulations relevant to your business.
- AI-Based Initial Filtering: The first AI agent evaluates whether a regulation is applicable.
- Applicable: The system marks it as relevant and extracts requirements.
- Non-Applicable: Users can manually review or add these to their library later.
- Efficiency: This step prevents information overload and ensures only actionable regulations are flagged for further analysis.
3. Building a Regulation Library
All relevant regulations are organized in a Regulation Library, with extracted requirements broken down for granular management:
- Requirement-Level Tracking: Each regulation is parsed into individual requirements for clear visibility and actionable oversight.
- Impact Assessment Agent: Determines which requirements directly apply to your business operations.
- Manual Overrides: Users can adjust applicability, mark requirements as non-relevant, or add notes for internal workflows.
- Centralized Access: Provides a single reference point for all regulatory data, supporting audits and compliance reporting.
4. Comparing Regulations
IONI enables comparison between different versions of a regulation or across jurisdictions:
- Version Comparison: Highlights:
- New Requirements – previously absent requirements now introduced.
- Changed Requirements – modified wording, obligations, or structure.
- Repealed Requirements – removed or restructured provisions.
- Cross-Jurisdiction Comparison: Compare regulations from different countries or with specific standards and directives to identify differences.
- Detailed Relations: The system links changes to specific regulation sections, helping users quickly understand context and implications.
5. Research and Analysis
Beyond structured comparisons, IONI’s Research Agent supports broader regulatory inquiries:
- Searches across the entire regulation database.
- Provides citations and references to relevant documents.
- Suitable for general questions or targeted investigations (e.g., “Overview of changes in EU medical device regulations”).
- Complements the requirement-level comparison by offering high-level insights for strategic decision-making.
6. Gap Analysis
Gap analysis ensures your internal documentation aligns with regulatory obligations:
- Comparison: Evaluates your company policies, procedures, and documents against applicable regulations.
- Identification: Flags missing or partially implemented requirements.
- Actionable Recommendations: Suggests either updating existing documents or creating new ones.
- Impact Assessment: Determines whether specific gaps apply to your business context.
- Iterative Improvement: After implementing recommended changes, you can re-run the analysis to confirm compliance.
7. Document Management and AI Assistance
IONI integrates document handling directly into the regulatory workflow:
- Upload & Conversion: Accepts PDFs, DOC, DOCX, and other formats, converting them to digital text for AI processing.
- Document Generation: Creates new documents from scratch based on gap analysis recommendations, aligned with your company’s context.
- AI-Driven Modifications: Suggests changes to existing documents to address gaps, allowing users to review, apply, or adjust recommended edits.
- Version Control: Tracks changes over time, maintains document history, and allows restoration to previous versions.
- Metadata & Tagging: Enables organization, filtering, and classification of documents for easy retrieval.
- Export Options: Documents can be exported in PDF or editable formats for external use or audit purposes.
8. Continuous Iteration and Horizon Scanning
A regulatory intelligence process is iterative:
- Regularly monitor sources for new or updated regulations.
- Apply AI agents for filtering, impact assessment, comparison, and gap analysis.
- Update or create documents in response to detected gaps.
- Maintain an organized and version-controlled library for audit readiness.
- Repeat the cycle to ensure ongoing compliance as regulations evolve.
Outcome:
By following this structured process in IONI, beginners in regulatory intelligence 101 can:
- Systematically capture and manage regulatory updates.
- Assess the applicability and impact of each requirement.
- Maintain a dynamic, compliant documentation base.
- Reduce the risk of missing obligations or compliance gaps.
Start building your regulatory intelligence process today with IONI and transform how your organization manages compliance. Book a demo, set up your regulatory sources, and see how AI agents can streamline monitoring, gap analysis, and document management - ensuring your company stays ahead of regulatory changes.
The Future of Regulatory Intelligence in Pharma
Staying compliant with constantly evolving regulations is a core challenge in the pharmaceutical industry. Regulatory intelligence 101 has traditionally relied on manual monitoring of agency websites, guidance documents, and industry publications. However, the growing volume and complexity of global regulations are pushing companies to adopt more advanced, technology-driven approaches.
The future of regulatory intelligence in pharma is increasingly shaped by AI, machine learning, and advanced analytics, enabling companies to track regulatory changes, inspection findings, and industry trends across the globe with unprecedented speed and precision.
AI Agents as the Core of Future Regulatory Intelligence
AI agents represent the next evolution in regulatory intelligence. Each agent is designed for a specific function, such as:
- Monitoring Agents: Continuously scan multiple regulatory sources, FDA databases, guidance documents, and public reports to detect new or updated requirements.
- Applicability Agents: Evaluate whether newly detected regulations or updates are relevant to a company’s specific operations, products, or markets.
- Impact Assessment Agents: Analyze how changes in regulations might affect processes, documentation, or product compliance.
- Gap Analysis Agents: Compare internal policies and documentation against regulatory requirements to identify missing or partially implemented obligations.
- Comparison Agents: Automatically compare current regulations with previous versions or across jurisdictions to identify changes, new requirements, or repealed rules.
- Research Agents: Answer complex queries, generate summaries, and provide citations across large volumes of regulatory content.
These AI agents operate continuously, providing near real-time updates and actionable insights.
Integration with AI-Powered Platforms
By integrating multiple AI agents into a centralized platform, pharmaceutical companies can:
- Create a dynamic regulatory library where all relevant regulations are tracked and continuously updated.
- Maintain a document base aligned with regulatory requirements, with AI agents suggesting updates or creating new documents as needed.
- Automate repetitive and time-consuming tasks, freeing staff to focus on high-level strategy and decision-making.
- Generate risk dashboards that highlight compliance gaps, regulatory trends, and potential areas requiring immediate attention.
AI in Practice: Real-World Applications
Pharmaceutical companies are already leveraging AI for regulatory intelligence with tangible results:
- NLP and data-mining systems monitor FDA databases, guidances, inspection findings, and public documents for regulatory changes.
- Centralized “data lakes” integrate internal quality data (deviations, CAPAs, risks) with external regulatory intelligence. AI extracts key concepts, relationships, and even sentiment - detecting negative regulatory trends.
- AI-powered dashboards provide real-time compliance risk indicators and recommendations, enabling proactive management across product formulation, supply chain, and regulatory compliance activities.
- Regulatory Q&A chatbots, powered by large language models, allow teams to ask natural language questions and receive summarized, actionable answers. These assistants can categorize regulatory changes by risk and highlight specific requirements for compounds.
- Semi-automated monitoring reduces hundreds of staff hours per month and ensures continuous compliance with FDA rules, guidance updates, and inspection trends.
- Pattern-recognition algorithms identify deviations or documentation errors that humans might miss, strengthening inspection readiness and minimizing regulatory citations.
AI-powered regulatory intelligence and compliance monitoring are elevating regulatory affairs into a strategic capability, enabling pharmaceutical companies to accelerate innovation, optimize resources, and maintain strong confidence in global compliance. Are you ready for the future of regulatory intelligence?
Conclusion: Turning Regulatory Intelligence 101 Into a Competitive Advantage
Regulatory intelligence 101 is a strategic advantage for pharmaceutical companies operating in an increasingly complex global environment. From understanding what regulatory intelligence is and why it matters, to exploring how it fits into the drug development lifecycle, organizations now recognize the critical role of systematic regulatory monitoring in ensuring safe, effective, and timely delivery of pharmaceutical products.
The essential components of regulatory intelligence - including monitoring global regulations, evaluating applicability, conducting impact assessments, and maintaining a centralized regulatory library - form the backbone of an effective compliance strategy. By integrating regulatory intelligence into every stage of the drug development process, companies can anticipate changes, reduce approval delays, and make informed decisions that safeguard both patient safety and business performance.
Pharmaceutical teams, regulatory affairs professionals, and compliance officers rely on regulatory intelligence tools and resources - ranging from industry news subscriptions to AI-powered platforms like IONI - to stay updated on standards, guidance documents, and regulatory trends. Beginners can leverage these tools to streamline processes, avoid common mistakes, and build a solid regulatory intelligence workflow tailored to their business. Step-by-step processes such as sourcing regulations, filtering for applicability, conducting gap analyses, comparing requirements, and managing documentation allow organizations to operationalize regulatory intelligence efficiently.
Ultimately, companies that embrace regulatory intelligence 101 as a core part of their strategy gain a competitive edge. They can maintain compliance across multiple jurisdictions and optimize resources, improve decision-making, and ensure a stronger, more resilient regulatory posture.
Are you ready to turn regulatory intelligence 101 into your competitive advantage?


.png)
.png)





