
Introduction
A product recall exposes how fragile even the strongest quality systems can be. One missed alert, one incomplete record, and what should be a controlled process becomes a public issue.
In 2026, companies are no longer looking for basic tracking tools. Instead, they need recall management software that integrates compliance, communication, and traceability in real time.
The search begins after a close call: a mislabeled batch, an unexpected supplier issue, or a customer complaint that triggered an internal investigation. These moments expose the limits of spreadsheets and manual coordination. Product recall management services now define how fast and precisely a company can respond when product integrity is at risk.
Recall activity continues to rise. In the U.S., more than 3,200 recall events were logged across five key industries. This is a high level compared to recent years. In Europe and the UK, recall events across five major industries reached almost 3,900 in a single quarter and crossed 7,700 in the first half of the year. These figures reflect heightened regulatory scrutiny and growing complexity in product safety and supply-chain traceability.
This guide reviews the best recall management software solutions of 2026, built for teams that need accuracy, accountability, and confidence when it matters most. It will help you navigate what matters, how recall management software and recall management services differ, and what you should demand to safeguard your brand and operations.
What is Recall Management Software?
After understanding why recall events are rising and why speed matters, the next step is clarity - what recall management software actually does and how it differs from recall management services.
Recall management software is a digital system that centralizes every element of a product recall. Typical functions include:
- Traceability of batches, lots, and serial numbers through production and distribution channels.
- Automated alerts and notifications to internal teams, suppliers, distributors, and customers.
- Centralized dashboards with real-time monitoring of recall status and response metrics.
- Integration with ERP/quality systems to link recall data with production, inventory, and supplier records.
- Documentation and reporting tools to satisfy regulatory requirements and audits.
- Scenario planning and mock recall simulations to test readiness.
The goal is precision and control across all stages of recall management: from initial detection to closure.
In contrast, recall management services focus on execution and logistics rather than technology, typically encompassing:
- Development or review of the recall plan and readiness programs.
- Risk assessment, coordination with regulatory authorities, and case‐by‐case management.
- Removal, retrieval, storage, or disposal of affected products from the supply chain or market.
- Communication with consumers, retailers, and distributors to manage returns, replacement,s or remediation.
- Post-recall analysis, root cause investigation, and corrective action support.
These services complement the software but rely heavily on manual processes and third-party coordination.
The difference becomes clear when looking at specific industries. In food manufacturing, recall management software connects directly to production and traceability systems, allowing rapid isolation of contaminated lots or mislabeled products.
Product recall management services then handle the removal and reporting, ensuring compliance with retailers and food safety authorities.
In pharmaceuticals, the software monitors serialized data, automates notification to distributors and healthcare providers, and maintains precise documentation for regulatory audits, while recall management services oversee product collection and verification.
Together, these solutions form a complete recall management framework - software for control and documentation, services for execution and recovery.
How to Choose Recall Management Software?
The next step is selecting the right solution. The choice depends on how your business manages traceability, compliance, and supplier communication, but also on how prepared you are to handle a recall when every decision counts.
When evaluating recall management software, focus on capabilities that strengthen product recall management and long-term reliability:
- End-to-End Traceability: The system should connect raw materials, production, and distribution data in real time. Automated batch and lot tracking are essential for instant product identification.
- AI-Powered Detection and Analysis: Modern recall management software uses AI to identify emerging risks, detect irregularities in quality or labeling data, and predict potential recall triggers before they escalate.
- Automated Alerts and Notifications: AI can prioritize alerts and route them to the right personnel instantly, cutting down response times during recall management.
- Integration with Quality and ERP Systems: Effective recall management depends on connected data. Choose a platform that integrates with production, inventory, and supplier systems to maintain accuracy across the process.
- Regulatory and Audit Readiness: Product recall management services rely on traceable documentation from the software. Ensure it produces real-time, audit-ready reports aligned with FDA, CFIA, and EU regulations.
- Simulation and Testing Tools: The best recall management software includes AI-assisted mock recall simulations that test readiness, identify weak points, and recommend corrective actions.
- Scalability and Multi-Site Oversight: Operations across multiple facilities need centralized visibility with site-level control. The platform should adapt as your organization grows.
- Data Security and Compliance: Protect sensitive records with encrypted data storage, access control, and AI-driven anomaly monitoring to detect unauthorized access.
- Vendor Expertise and Support: The strongest providers offer both software and product recall management services, combining digital capability with operational guidance.
AI has become a defining feature of modern recall management. It moves recall management software forward, helping teams identify risks early, streamline decision-making, and maintain control before disruption spreads.
Best Recall Management Software
After defining what recall management software should deliver and what to ask vendors, the next step is reviewing the leading solutions available today. Each platform below is designed to simplify recall management, strengthen traceability, and support compliance across industries such as food, beverage, and pharmaceuticals.
While some focus on technology and automation, others combine digital tools and AI with product recall management services for full operational support.
#1 IONI
IONI is an AI-powered recall management software designed specifically for the food industry. It connects quality, safety, and production teams through a fully automated workflow that transforms how businesses handle deviations and manage recalls.
By combining AI-driven risk detection, CAPA automation, and real-time compliance monitoring, IONI delivers a complete product recall management solution for manufacturers, processors, and co-packers.
How IONI works:
Step 1. Detection and Recall Workflow Activation
IONI’s recall management process begins when deviations appear in production, quality, or storage data, such as temperature fluctuations, contamination risks, or process inconsistencies. Once detected, the system automatically logs the event, assigns a risk level, and triggers the recall workflow. Deviations across multiple facilities or lines are tracked in one place, linking them directly to recall cases to ensure immediate containment and traceability.
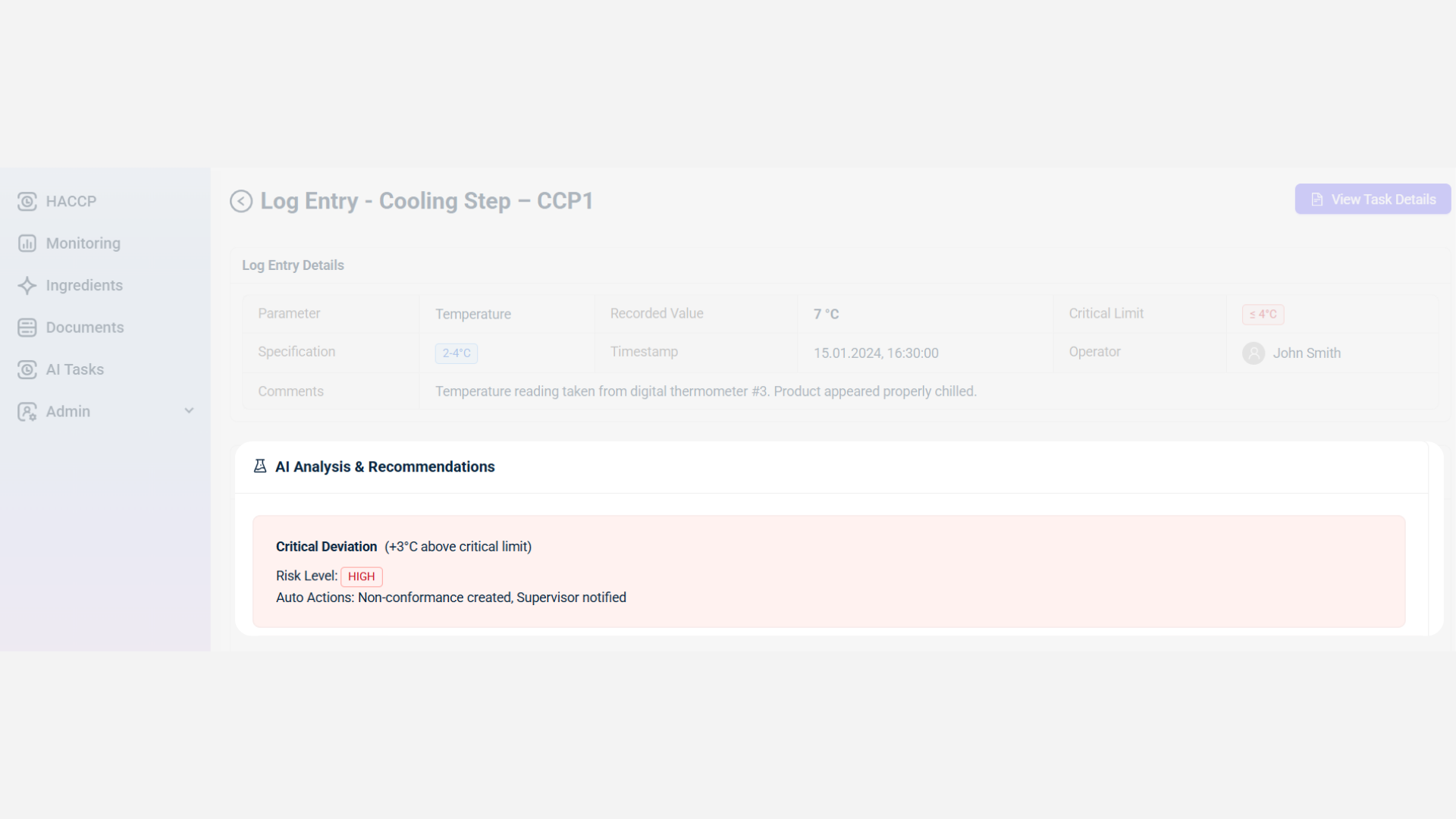
Step 2. AI-Suggested Corrective & Preventive Actions (CAPA)
IONI applies AI to recommend corrective and preventive actions tailored to the deviation’s severity. Suggested actions include quarantining affected batches, recalibrating equipment, or revising SOPs. Preventive recommendations often include additional monitoring points, maintenance scheduling, or staff training.
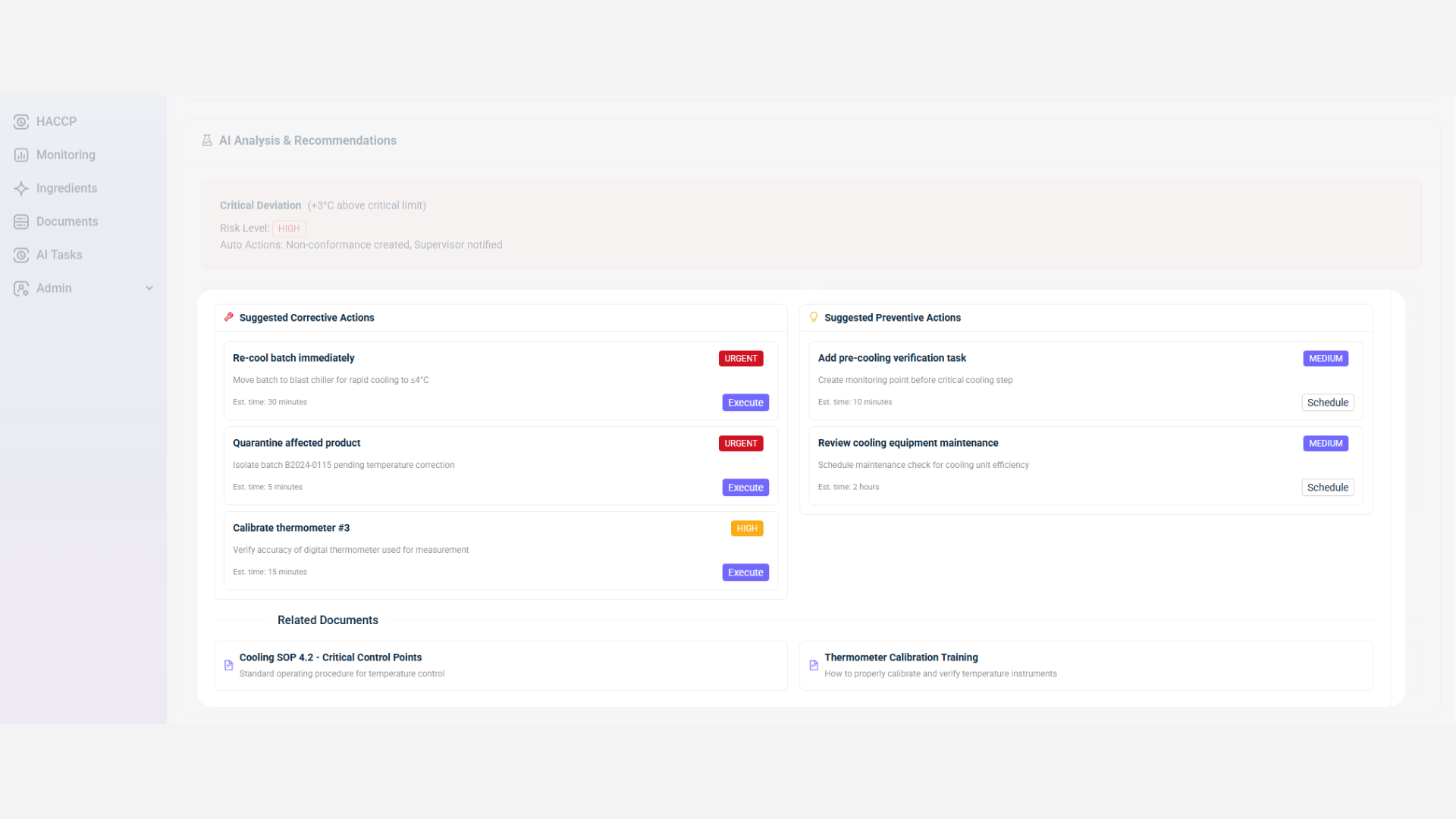
Step 3. QA Manager Oversight
Once AI suggestions are generated, the QA Manager reviews, approves, or modifies them based on operational judgment. Actions are executed and tracked within the system, creating a clear audit trail. Non-conformances (NCs) and CAPAs are assigned to specific team members, ensuring accountability and verification of completion.
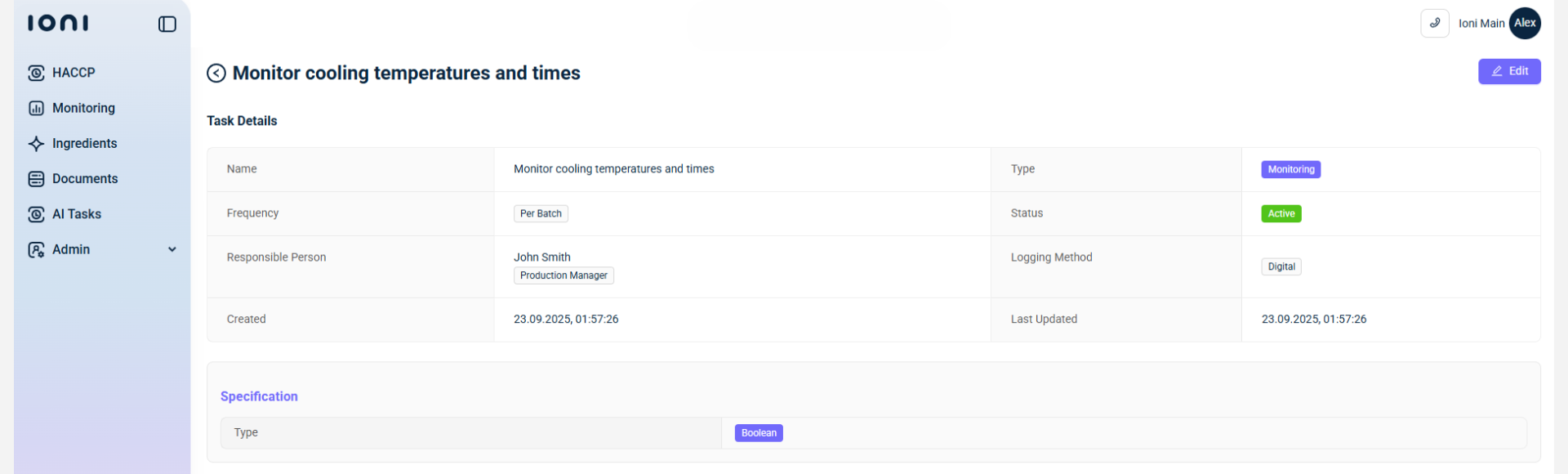
Step 4. SOP and Documentation Updates
Preventive actions feed directly into SOP and documentation updates. IONI’s recall management software centralizes all recall-related records: SOP versions, calibration logs, verification reports, ensuring full traceability and audit readiness. Updated documentation automatically integrates into staff training and compliance workflows.
Step 5. Task Management and Tracking
IONI converts CAPA actions into structured tasks with deadlines, descriptions, and priority levels. Each task is assigned to responsible personnel and tracked in real time. Whether it’s reprocessing, verification, or preventive maintenance, the platform ensures that every corrective measure is executed without delay.
Step 6. Compliance Dashboard
The unified dashboard provides an instant overview of all recall management services and food safety compliance metrics:
- Active deviations and nonconformances
- Open and closed CAPAs
- Task completion status
- SOP and training progress

This enables management teams to monitor performance, detect bottlenecks, and maintain regulatory readiness while generating detailed reports for internal and external audits.
Step 7. AI Insights and Risk Analysis
IONI’s AI engine continuously analyzes data to detect patterns and predict risks. It identifies recurring deviations, missing SOP updates, or regulatory gaps, and generates predictive alerts for potential process or equipment failures.
Explore how IONI’s recall management software and product recall management services can help your organization manage recalls faster, smarter, and more reliably.
#2 FoodLogiQ by Trustwell
FoodLogiQ is another recall management software purpose-built for the food industry, focusing on visibility, speed, and traceability across the entire supply chain. Unlike generic product recall management solutions, FoodLogiQ integrates directly with food production and distribution data, giving brands the tools to respond to contamination, mislabeling, or allergen risks with precision.
Key Capabilities:
- Real-time recall tracking and communication: Centralized dashboards display recall progress and automate notifications via email, text, and phone, ensuring immediate awareness across all supply chain partners.
- Lot- and batch-level traceability: Enables quick identification and removal of affected product lines without disrupting unaffected stock.
- Mock recalls and withdrawal testing: Built-in simulation tools allow food manufacturers to test recall readiness and strengthen protocols.
- Regulatory documentation: Automatically generates detailed reports and audit trails to meet FDA, CFIA, and global food safety compliance requirements.
- Integration with supplier management systems: Maintains supplier records, compliance data, and recall response times for each partner.
FoodLogiQ suits mid- to large-scale food manufacturers and brands managing complex supplier networks or distributed production. It provides a structured, auditable recall process backed by traceability data and integrated communication.
#3 ECRI
ECRI’s automated recall management software is a trusted solution in healthcare and pharmaceuticals, recognized for its precision and compliance in high-risk, regulated environments. It centralizes the recall process - from alert detection to resolution - helping businesses minimize patient risk and operational disruption.
Key Capabilities:
- Automated recall alert matching: ECRI’s Automatch technology identifies which recalled items are actually in inventory, ensuring immediate, targeted action.
- Early recall notifications: Users receive alerts often before official regulatory publication, gaining valuable time to respond.
- Workflow automation: Tasks, responsibilities, and verification steps are assigned automatically across teams, reducing administrative effort.
- Comprehensive audit trails: Every step - from initial alert to closure - is documented to support compliance with FDA and Joint Commission standards.
- Enterprise integration: Works seamlessly with ERP and hospital inventory systems, including Workday, for full visibility and coordination.
ECRI has decades of experience in medical device safety and recall research. The platform’s strength lies in its ability to filter out irrelevant alerts and focus on what directly affects patient care. Automating identification, communication, and documentation reduces recall response time and supports hospitals in maintaining uninterrupted, compliant operations.
#4 Qualityze
Qualityze’s solution supports full lifecycle management of safety actions and product recall events. It’s positioned as a tool to handle deviation detection, corrective action workflows, communications, and regulatory reporting - all elements relevant to recall management.
Key Capabilities:
- Centralized tracking of field-safety actions (FSAs) and recall events, including logged issues, distribution data, and status updates.
- Health hazard analysis (HHA) tools to evaluate the risk of product issues and classify severity.
- Notification workflows for affected parties and regulatory bodies; integration of communications channels.
- Traceability of affected batches/lots across distribution networks; linking issues to recall actions.
- Integration with other quality management modules: CAPA, Nonconformance, Supplier Quality, etc.
- Configurable workflows and templates tailored for complex regulatory environments.
- Cloud-based architecture designed for scalability and centralized oversight across sites.
- Focus on compliance with regulations such as ISO 10377, FDA reporting timelines, and consumer safety frameworks.
This platform supports recall management by providing traceability, automated workflows, and compliance-ready documentation.
#5 AmpleLogic
AmpleLogic offers a module for product recall management within its broader electronic quality management system (eQMS). Its recall-focused module supports key recall workflows, making it a viable candidate for companies looking at recall management software.
Key Capabilities:
- Dedicated “Product Recall” module within the eQMS suite, enabling recall event tracking and lifecycle management.
- Integration with CAPA, change control, deviation, and audit modules—providing a unified platform for product recall management alongside other quality processes.
- Support for regulatory compliance standards such as 21 CFR Part 11 and EU Annex 11, increasing readiness for audit and traceability requirements.
- Low-code/no-code platform design enabling faster setup and customization to specific process flows and risk profiles.
- Dashboards and reporting features that allow for monitoring recall status, tasks, and audit-trail visibility.
- Modular architecture allows users to configure recall workflows and link them dynamically with CAPA, deviation, supplier quality, and more.
- Customization and rapid deployment: the low-code platform suggests reduced implementation timelines compared with more rigid systems.
AmpleLogic is designed for regulated environments where recalls must be managed within a validated quality framework. Its integrated structure makes it suitable for businesses seeking full oversight of recall activity alongside other quality and compliance operations.
#6 TrackWise by Honeywell
TrackWise Recall Management provides an end-to-end software solution for handling product recalls or withdrawals. It covers the lifecycle from signal detection through decision-making, execution, tracking, and regulatory reporting. Built for pharmaceutical and medical device environments with high demands for documentation and compliance.
Key Capabilities:
- Signal detection and decision-tree frameworks for scope and impact assessment of recall events.
- Batch/lot identification, notification preparatio,n and consignee list generation for effective communication.
- Automated integration with enterprise systems (QMS, RIMS, PLM, ERP) to consolidate recall data and streamline workflows.
- Audit-ready reporting, eSignatures, and full traceability to support regulatory compliance.
- AI-enabled analytics: The latest version introduces AI-assisted recall detection and execution to reduce cycle time.
TrackWise Recall Management offers a robust platform for organizations that need formalized workflows, strong audit trails, and regulatory alignment in their recall management processes. It suits enterprises that require both recall management software and a system capable of contributing to or coordinating with product recall management services.
Conclusion
Effective recall management is a data-driven discipline that defines how resilient and transparent a business truly is. Modern recall management software enables companies to detect risks faster, coordinate corrective actions in real time, and maintain full regulatory traceability.
While recall management services provide external support during high-pressure events, internal deployment of product recall management software ensures control and consistency across all stages: from issue identification to market withdrawal and post-recall analysis. Many companies now combine both approaches: using product recall management services for logistics and communications, and relying on digital platforms to handle automation, documentation, and compliance.
The evolution of recall management points toward AI-driven systems that predict potential failures and initiate proactive containment. For food, pharma, and other regulated sectors, adopting the right digital infrastructure is essential to maintain brand integrity and public trust.
In short, the most effective strategy is a connected one: pairing robust recall management software with well-defined recall management services to build a system capable of responding instantly, learning continuously, and preventing the next recall before it starts.
FAQ
How does recall management software integrate with ERP or quality management systems (QMS)?
Recall management software integrates directly with ERP and QMS platforms to synchronize production, traceability, and quality data. This integration allows automatic linkage between batch records, supplier information, and deviation reports. When a recall event is triggered, affected SKUs, suppliers, and customer records are identified instantly.
How does recall management software handle supplier-related or cross-border recalls?
Modern recall management software centralizes supplier data, certificates, and traceability documentation. When a supplier issue emerges, the platform maps ingredient flow through all finished products, identifying exposure across facilities and regions. For cross-border recalls, it aligns with multiple regulatory frameworks, ensuring localized notifications and reporting requirements are met.
What are the main regulatory requirements that recall management software helps to meet?
Recall management software supports compliance with FDA 21 CFR Part 7, CFIA recall protocols, and EU Regulation (EC) No 178/2002, among others. It automates documentation, timelines, and communication logs required for regulatory reporting. By linking product traceability, CAPA actions, and supplier records, the software ensures full audit readiness.
How secure is product data within recall management software platforms?
Leading recall management software platforms use encryption, access controls, and role-based permissions to protect sensitive product and supplier data. Cloud-based systems often include audit trails and activity monitoring to ensure data integrity during recall events.
Does AI in recall management software actually prevent recalls, or only speed up response time?
AI in recall management software serves both preventive and reactive roles. It identifies early risk patterns - such as repeated deviations, supplier performance issues, or temperature fluctuations - enabling businesses to act before a recall becomes necessary. During an active event, AI accelerates risk assessment, corrective actions, and communication, reducing overall recall time.


.png)
.png)





