
Introduction
Hazard Analysis and Critical Control Points (HACCP) is a globally recognized, science-based system for managing food safety. Instead of relying only on end-product testing, HACCP focuses on identifying potential hazards such as biological, chemical, or physical risks at every stage of the food production process, from sourcing raw materials to final distribution.
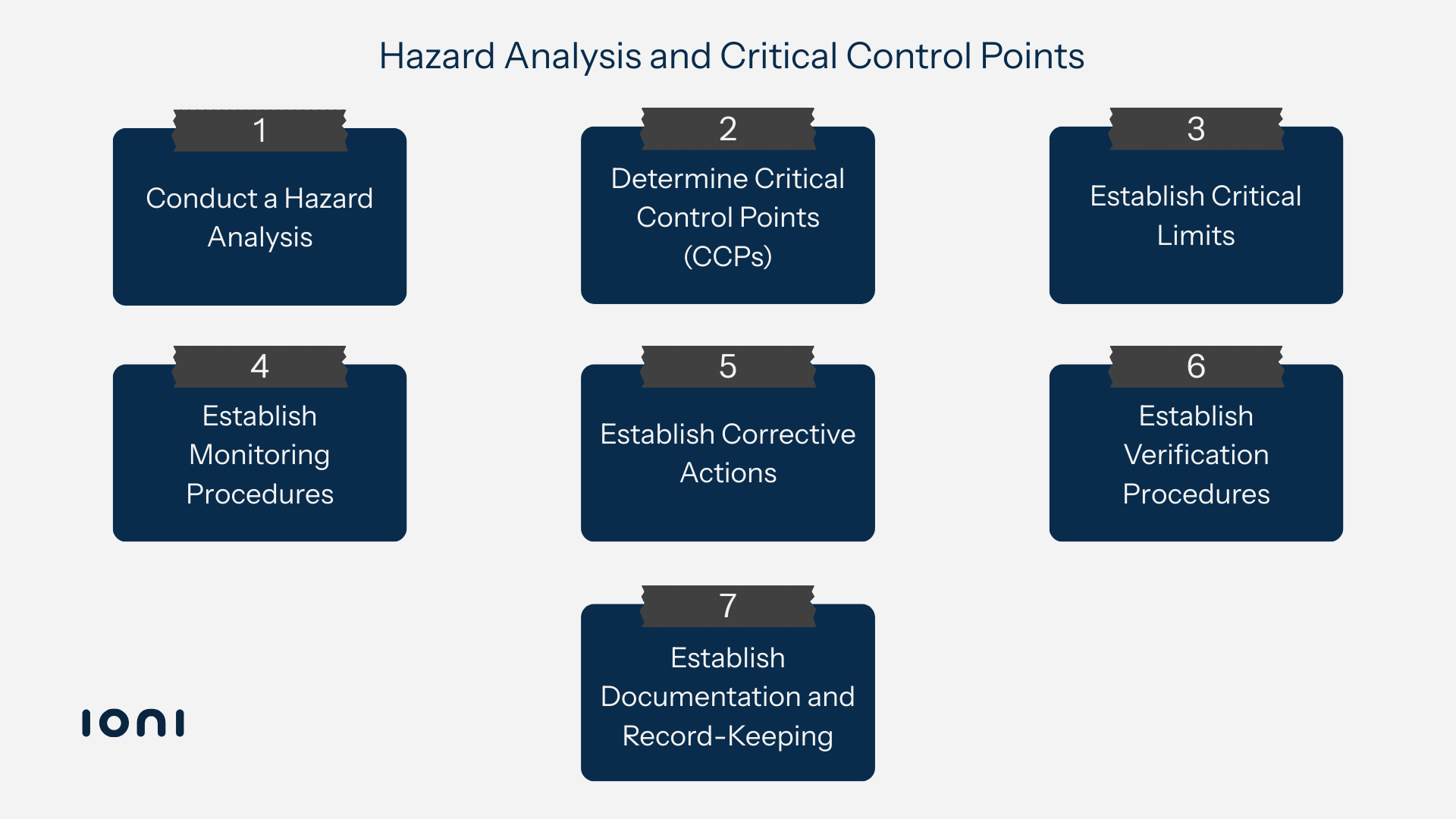
It pinpoints critical control points where hazards can be prevented, eliminated, or reduced to safe levels, enabling proactive risk management. This preventive approach ensures compliance with international food safety regulations, strengthens consumer confidence, reduces the likelihood of product recalls, and protects public health.
What Is a HACCP Audit and Why Does It Matter?
A HACCP audit is a formal evaluation of a company’s Hazard Analysis and Critical Control Points system to verify that it effectively controls food safety risks. Rather than simply confirming that procedures exist, the audit checks whether those procedures are correctly implemented, properly documented, and consistently followed in day-to-day operations.
The process plays a dual role: it safeguards public health and protects your business from regulatory penalties, product recalls, and reputational damage. During HACCP auditing, an auditor examines records, observes production areas, and interviews staff to confirm that each identified hazard is controlled at its designated critical point.
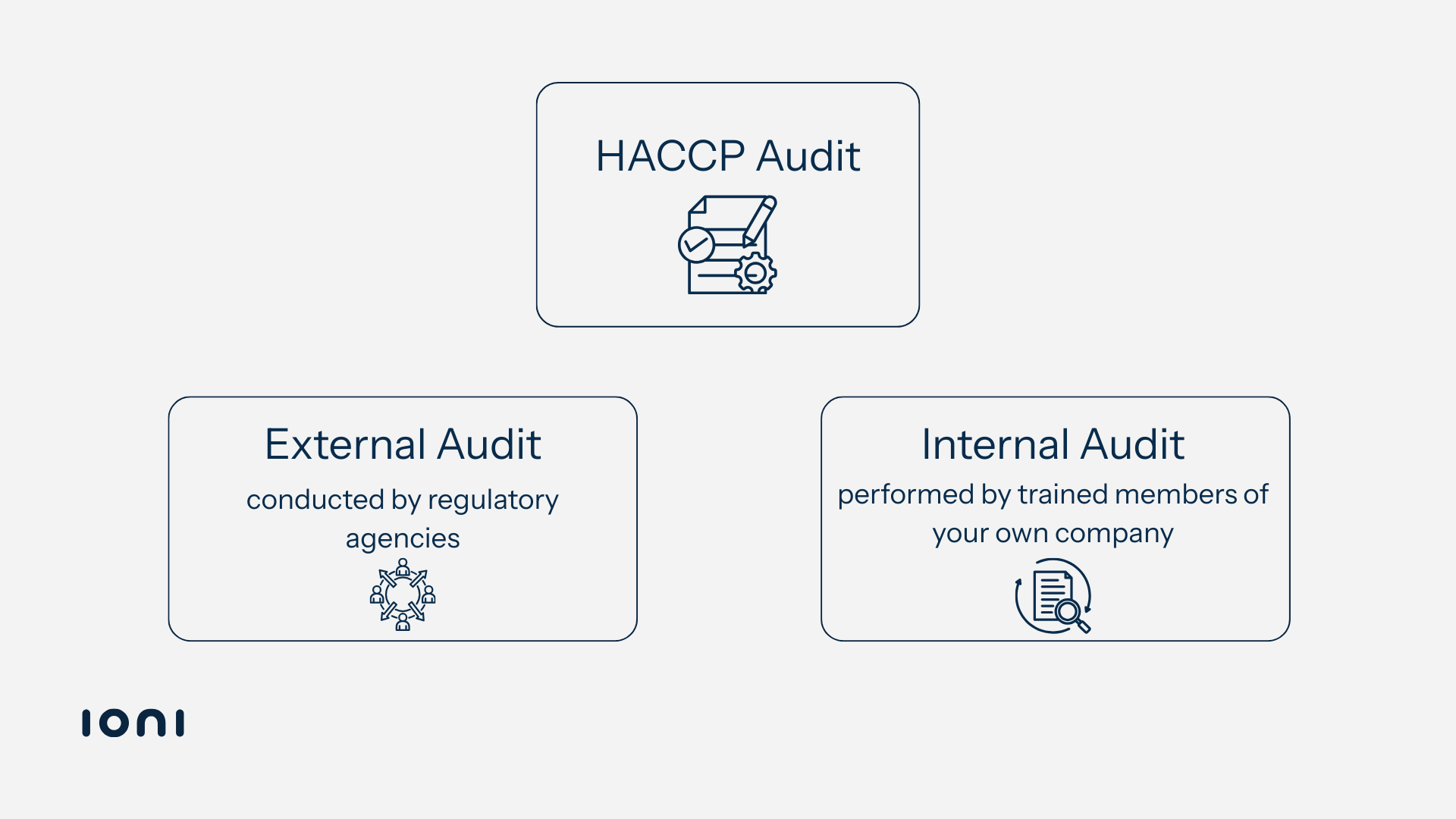
There are two primary types of HACCP audits:
- External Audits - conducted by regulatory agencies, certification bodies, or large clients that are not a part of the company being audited. These audits are often scheduled to meet legal requirements, industry standards (such as ISO 22000), or customer agreements. They typically have a high level of scrutiny, as results may affect your certification status or your ability to supply certain markets.
- HACCP Internal Audits - performed by trained members of your own company or appointed third-party consultants acting as internal evaluators. The goal is to assess compliance before an external inspection, identify non-conformities, and ensure corrective actions are in place.
HACCP audits are required when applying for certification, renewing an existing certification, meeting specific industry regulations, or fulfilling contractual obligations. However, businesses that go beyond the minimum requirements and schedule regular HACCP internal audits tend to maintain stronger systems. This approach reduces the risk of unexpected failures during an external audit and ensures the HACCP plan remains relevant as processes, equipment, or regulations change.
Understanding what a HACCP audit is and the distinction between internal and external reviews provides the foundation for effective preparation. In the following sections, we’ll break down how these two audit types differ in scope and purpose, who needs to comply, and the exact checklist items auditors expect to see. By the end, you’ll have a clear framework for conducting your own HACCP internal audit and staying fully prepared for any external inspection.
HACCP Internal Audit vs External Audit: Key Differences
Before delving into the specifics of the HACCP audit checklist, it's essential to understand the distinct purposes behind different types of HACCP auditing. Not all audits are created equal - and recognizing how internal and external reviews differ will help you apply the right strategy to the right context.
Why Does Distinction Matter?
Think of HACCP internal audit as your early-warning system: a proactive, in-house effort designed to uncover weak points in your food safety system before a formal evaluation. This internal check allows you to correct issues, strengthen documentation, and ensure that procedures genuinely reflect daily practice, all in a controlled, low-risk setting.
In contrast, a third-party HACCP audit - whether conducted by a regulatory body, a certification organization, or a client - serves as formal validation of your compliance with official food safety standards and legal requirements.
How to Distinguish Internal vs External Audits
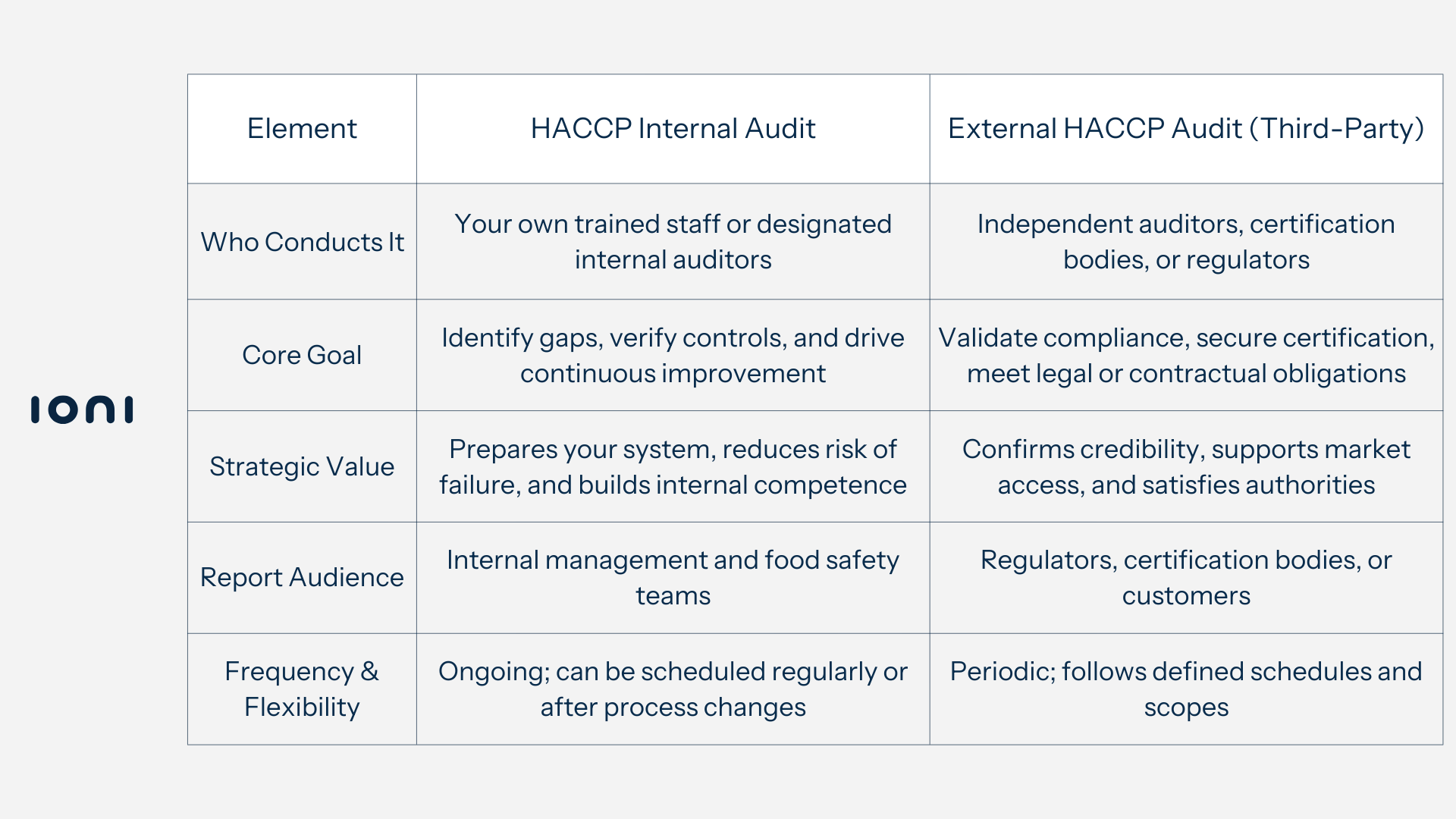
Structure & Conduct
A HACCP internal audit is usually performed by trained members of your organization - often food safety staff or appointed internal auditors. Their knowledge of your systems, workflows, and culture allows them to identify issues in context and offer practical solutions.
An external HACCP audit, on the other hand, is conducted by independent third parties, such as certification bodies or regulatory inspectors. External auditors bring objectivity and a compliance-focused perspective that is essential for formal assessments.
Goals & Value
The primary goal of a HACCP internal audit is proactive improvement: verifying that procedures are implemented as intended, identifying weaknesses, and ensuring corrective actions are in place before issues escalate.
The purpose of an external HACCP audit is to confirm that your food safety system meets all applicable regulatory, certification, or customer standards. These audits carry significant weight, as their results can determine certification status and compliance credibility.
Reporting & Audience
Internal audit findings are reported to management or internal teams, focusing on detailed insights and operational improvements.
External audit results are shared with regulators, certification bodies, or customers, and may directly affect your ability to maintain certification or continue business with certain clients.
How Internal Audits Support Compliance
Regular HACCP internal audits are a critical part of ongoing compliance. By systematically reviewing documentation, records, and procedures, internal teams can spot non-conformities early, apply corrective actions, and ensure the HACCP plan stays aligned with current operations and regulations.
This routine oversight helps prevent costly failures during external audits, strengthens internal accountability, and fosters a culture where food safety is embedded into daily operations - not just addressed when an inspection is on the horizon.
Who Needs to Pass HACCP Audits?
In the previous section, we compared HACCP internal audits and external HACCP audits, highlighting how they differ in purpose, structure, and value.
But before focusing on the step-by-step checklist, it’s worth asking: who actually needs to pass these audits, and how should they prepare? The answer is broader than many businesses realize.
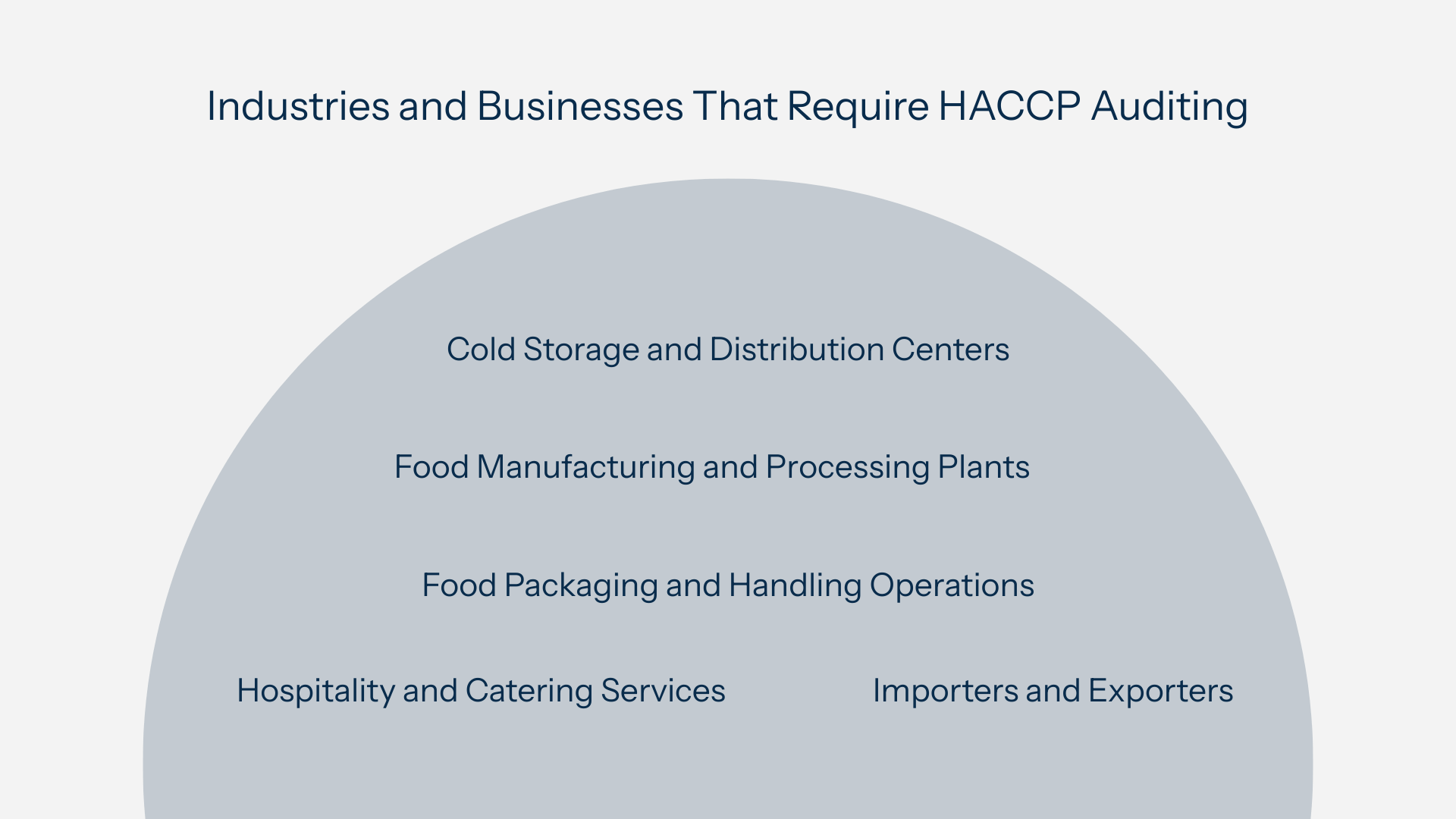
Industries and Businesses That Require HACCP Auditing
HACCP is not limited to large-scale food manufacturers. Any business involved in the handling, processing, packaging, storage, or distribution of food products can fall under audit requirements - either through legal obligations or customer expectations.
Key sectors include:
- Food manufacturing and processing plants - meat, poultry, seafood, dairy, beverage, and bakery production all require structured food safety systems and regular audits.
- Food packaging and handling operations - manufacturers of primary packaging (direct contact with food) are often audited to ensure materials don’t pose contamination risks.
- Cold storage and distribution centers - maintaining proper storage conditions and preventing cross-contamination during transport are critical audit points.
- Hospitality and catering services - restaurants, hotels, and institutional kitchens must demonstrate consistent control over food safety hazards.
- Importers and exporters - many countries require HACCP certification for food imports; failing an external audit can block shipments entirely.
Even if your local regulations do not explicitly require HACCP certification, market pressures often make it unavoidable. Large retailers, distributors, and corporate clients frequently require suppliers to pass a HACCP audit as part of their approval process.
Preparing for a HACCP Audit
Whether you’re undergoing an external review or conducting a HACCP internal audit, the same principles apply: preparation is the difference between a smooth approval and a costly non-conformance report.
Here are the key steps to prepare:
1. Align and train your team
Everyone who touches the HACCP system - from line operators to quality managers - should understand their responsibilities during the audit. This includes knowing where records are stored, being able to explain their tasks, and demonstrating correct procedures in practice.
2. Review and update documentation
Outdated or incomplete records are one of the most common reasons for audit findings.
Verify that:
- The HACCP plan is current and reflects actual operations.
- Standard Operating Procedures (SOPs) are up to date and signed.
- Monitoring logs, verification records, and calibration certificates are complete.
- Corrective action reports are documented with evidence of resolution.
3. Identify common gaps before the auditor does
A pre-audit or structured HACCP internal audit is the best tool for spotting issues early. Frequent problem areas include missing verification records, incorrect Critical Control Point (CCP) limits, incomplete training logs, and a lack of documented supplier approvals.
Common gaps include:
- Incomplete or outdated hazard analysis - critical steps, raw materials, or new processes are missing from the hazard identification document.
- Incorrect or unverified Critical Control Point (CCP) limits - CCP limits aren’t based on scientific data or haven’t been validated.
- Missing monitoring records - temperature logs, pH checks, or other monitoring documentation are incomplete or have missing entries.
- Inadequate corrective action records - issues are recorded but lack evidence of resolution or follow-up verification.
- Expired or missing supplier approvals - approved supplier lists are outdated or lack supporting documentation such as audit results or COAs.
- Gaps in employee training - training records are missing, outdated, or do not match the employee’s current role and responsibilities.
- Poor document control - multiple versions of procedures exist, with no clear indication of the current approved version.
- Inconsistent implementation - written procedures differ from what is actually practiced on the production floor.
4. Ensure traceability and recall readiness
Auditors often test your traceability system by asking you to track a product from raw material to finished goods - or vice versa. This should be achievable quickly and with clear documentation.
5. Simulate audit conditions
Conduct a mock audit where an internal auditor walks the site, reviews records, and interviews staff. This reveals gaps and helps the team feel comfortable answering questions under audit conditions.
The Role of Internal Audits in Readiness
A well-planned HACCP internal audit acts as a dress rehearsal for the real thing. By addressing issues in advance, you minimize the risk of surprises during an external assessment. This proactive approach protects your compliance status, avoids costly delays, and strengthens customer confidence in your operations.
The Complete HACCP Audit Checklist
Having identified who needs to pass a HACCP audit and the most common pitfalls, the next step is to turn preparation into a structured, repeatable process. A clear checklist ensures that nothing is overlooked - whether you are conducting a HACCP internal audit or preparing for a third-party assessment.
Auditors evaluate the required documentation that exists and is current, complete, and implemented in practice. Below is a step-by-step breakdown of the core documents, records, and procedures that HACCP auditing typically covers.
1. HACCP Plan and Hazard Analysis
Your HACCP plan is the backbone of the audit. Auditors will expect:
- A current, approved copy of the HACCP plan with version control.
- A comprehensive hazard analysis covering all biological, chemical, and physical hazards for each process step.
- Documented rationale for identifying or rejecting potential hazards.
- A list of Critical Control Points (CCPs) with clearly defined limits, monitoring methods, and corrective actions.
- Flow diagrams that accurately represent current operations, equipment layout, and process steps.
A document register listing all documents referenced in the HACCP system, which may include:
- HACCP team composition
- Product description and intended use
- Hazard analysis, including risk assessment and scientific references
- CCP determination
- Critical limit validation
- HACCP audit table
- Specifications for raw materials and finished products
- Formulations (recipes)
- Pre-requisite programmes
- Standard operating procedures and work instructions
- Policies
Detailed Product Descriptions
For each product or group of products, a thorough product description must include:
- Composition (formulation/ingredients)
- Physical and chemical characteristics (e.g., aW, pH, preservatives)
- Production methods and technologies (e.g., heat treatment, HPP, freezing, drying, brining)
- Primary and secondary packaging (type, durability, shelf-life effects)
- Storage, handling, and distribution methods (e.g., refrigerated or ambient transport)
- Shelf life, including best-before or use-by date coding
- Intended use of the product(s)
- Labeling requirements and claims per local legislation
- Allergen content per local legislation
- Identification of sensitive consumers
2. Standard Operating Procedures (SOPs)
SOPs must be complete, relevant, and aligned with actual on-floor practices. Expect auditors to review:
- Written SOPs for cleaning and sanitation, equipment operation, maintenance, storage, allergen control, and personal hygiene.
- Document control showing issue dates, review dates, and signatures from responsible personnel.
- Evidence that SOPs are implemented exactly as written - auditors may observe processes to confirm compliance.
- Corrective actions were taken when SOP deviations occurred.
3. Critical Control Point (CCP) Monitoring Records
- CCP monitoring is a key focus in HACCP auditing. You’ll need:
- Daily monitoring logs for each CCP, with dates, times, and signatures of responsible staff.
- Clear documentation of corrective actions when CCP limits were exceeded.
- Verification that monitoring records are reviewed regularly by a supervisor or manager.
- Consistency between recorded values and calibration of monitoring equipment.
4. Verification and Validation Records
Auditors will confirm that your HACCP plan has been both validated and routinely verified:
- Validation records showing that CCP limits are scientifically sound and meet regulatory or industry requirements.
- Internal verification reports, such as HACCP internal audit results, with follow-up actions documented.
- Calibration certificates and records for thermometers, pH meters, scales, and other monitoring tools.
- Microbiological or chemical test results are used to verify food safety controls.
5. Traceability and Recall Procedures
Traceability is a critical requirement during a HACCP audit:
- Ability to track ingredients from the supplier to the finished product and distribution.
- Documentation of supplier approvals, including Certificates of Analysis (COAs), inspection results, and supplier audit reports.
- Records of mock recalls or traceability exercises, showing start-to-finish tracking within the target time frame.
- Defined recall procedures with assigned responsibilities and communication protocols.
6. Training Records
Auditors will check whether personnel are trained, competent, and up to date:
- Training logs for all employees involved in HACCP-related activities, with dates, content, and trainer signatures.
- Job-specific training records (e.g., CCP monitoring, sanitation procedures, allergen handling).
- Refresher training schedules and evidence of retraining after significant process or product changes.
- Competency assessments, such as quizzes, practical demonstrations, or observation checklists.
7. Facility and Equipment Records
Facilities and equipment must be maintained to prevent contamination:
- Cleaning and sanitation records for all food-contact and high-risk areas, with chemical usage documented.
- Preventive maintenance logs for equipment critical to food safety, including service dates and technician notes.
- Pest control contracts, inspection reports, and corrective action records.
- Environmental monitoring results (if applicable), showing control of pathogens in the facility.
Pro Tip: Use this expanded checklist during a HACCP internal audit to identify gaps before an external auditor arrives. Document each review, note corrective actions, and keep evidence ready - this proactive approach often means the difference between a smooth certification and a long list of non-conformances.
Conducting a HACCP Internal Audit Step-by-Step
After completing The Complete HACCP Audit Checklist, the next stage is to put that framework into action through a structured HACCP internal audit. This process verifies that your HACCP plan works as intended, identifies gaps before they become risks, and ensures your food safety system continues to meet both regulatory and customer expectations.
Below is a practical, standards-aligned approach to HACCP auditing.
1. Plan Your Audit Thoroughly
- Define the scope - products, processes, and sites to be reviewed.
- Set objectives - confirm compliance, assess effectiveness, and identify improvements.
- Assemble an audit team - ensure auditors are independent from the processes they assess.
- Prepare tools - your current HACCP plan, hazard analysis tables, CCP decision trees, GMP procedures, and previous audit reports.
- Schedule strategically - choose times when critical processes and CCP monitoring can be observed.
Tip: Ensure access to all internal and external documents that support your HACCP system, including regulatory standards, codes of practice, and supplier certifications.
2. Conduct a Pre-Audit Document Review
Before going on-site, check that:
- HACCP scope and purpose are clearly documented.
- Product descriptions and intended use are up to date and cover allergens, shelf life, packaging, and consumer groups.
- Process flow diagrams have been verified within the last 12 months.
- Hazard analysis includes all biological, chemical, physical, allergen, and (if relevant) radiological hazards.
- Critical limits are validated and scientifically supported.
- Monitoring and verification records are complete, signed, dated, and easily retrievable.
- Corrective actions show evidence of root cause analysis and follow-up checks.
3. Perform the On-Site Audit
During the HACCP auditing process:
- Observe operations - confirm CCP monitoring matches documented procedures and occurs at the defined frequency.
- Interview personnel - verify they understand the CCP they monitor, the critical limit, and the corrective actions required for deviations.
- Inspect records - check signatures/initials, timestamps, and corrective action logs for accuracy.
- Verify equipment calibration - ensure monitoring devices are accurate, calibrated, and documented.
- Assess GMP compliance - inspect hygiene practices, cleaning schedules, allergen management, pest control, supplier approval, and traceability.
- Cross-check controls - confirm that prerequisite programmes are implemented effectively and monitored regularly.
4. Record and Classify Findings
- Document both conformities and non-conformities.
- Reference the exact HACCP requirement breached for each non-conformity.
- Classify findings by severity:
- Critical - potential food safety hazard
- Major - system gap that could lead to a hazard
- Minor - isolated lapse without immediate safety impact
5. Require Corrective Actions and Follow-Up
Every non-conformity must include:
- Description of the issue and the HACCP criterion affected
- Assigned responsibility and completion deadline
- Root cause analysis to prevent recurrence
- Evidence of action taken and verified effectiveness
- Correction vs. corrective action clearly distinguished
- Evidence of completion and effectiveness verification (trend data, re-audit check, validation/verification update)
Tip: Monitor the status of all corrective actions in subsequent internal audits or management reviews to confirm closure.
6. Report to Management
Prepare a structured HACCP audit report covering:
- Scope, objectives, and audit criteria
- Summary of activities (document review, interviews, observations)
- Detailed findings and classifications
- Agreed on corrective actions and timelines
- Overall conclusion on HACCP system effectiveness
Management should review audit results promptly to ensure adequate resources for corrections and ongoing improvement.
7. Integrate with Continuous Improvement
Internal audits are not a one-time compliance exercise. Use results to:
- Update the HACCP plan when processes, products, or hazards change
- Strengthen training programmes where knowledge gaps are found
- Refine GMP procedures to prevent repeat findings
- Improve monitoring and verification methods based on audit evidence
- Feed trends (CAPA on-time rate, repeat NCs, CCP deviations) into management review and objectives.
A HACCP internal audit is a targeted tool for verifying that your food safety system performs as documented and stands up to real-world conditions. By combining document review, on-floor observations, and staff interviews, you gain a full picture of how controls operate in practice. Structured reporting, risk-based classification of findings, and disciplined corrective-action tracking ensure that every issue is resolved and that the system evolves with your products, processes, and regulations.
When approached methodically, HACCP auditing prevents non-conformities during external inspections and strengthens the culture of food safety across the business.
Common Reasons Businesses Fail a HACCP Audit
After mastering the process of conducting a HACCP internal audit, the logical next step is to understand why so many facilities still fail when it comes to an actual HACCP audit. Failures rarely happen because a business lacks a HACCP plan - they happen because the plan is incomplete, incorrectly implemented, or not supported by consistent evidence.
Discovering these pitfalls before a third-party auditor does is one of the main benefits of regular HACCP auditing. Below are the most frequent and damaging reasons businesses fail, backed by real-world examples and linked to specific HACCP principles or Codex/FDA requirements.
1. Hazard Analysis Gaps and Over-Simplification
Many businesses fail Principle 1 because hazard identification is incomplete or based on assumptions rather than documented evidence.
- Companies sometimes list “microbiological hazard” without specifying the pathogen or its source.
- Some hazard tables show “none” for chemical or physical hazards without justification - often because no review of supplier materials or industry alerts has been conducted.
- Allergen hazards are sometimes marked “not applicable” when shared facilities clearly introduce risk.
Example: In one audit, a catering business excluded Listeria monocytogenes as a hazard in ready-to-eat foods despite chilled storage and prolonged shelf life - contradicting guidance and breaching Codex Principle 1.
2. CCP Selection and Justification Failures
Principle 2 violations often occur when CCPs are chosen without a decision tree or risk-based reasoning.
- “Catch-all” CCPs are declared for almost every step, making monitoring impractical.
- Alternatively, genuinely critical steps (e.g., metal detection in the final product) are left as “GMP” controls with no critical limit.
- No explanation is given for CCP's choice, making it impossible for auditors to verify the reasoning.
Example: A fish processing site failed its audit because freezing for parasite destruction was listed as a prerequisite control - not a CCP - despite being the only control step for that hazard.
3. Unvalidated or Incorrect Critical Limits
Principle 3 requires limits based on science or regulation, but in practice:
- Some plans set “approximately” or “about” values rather than precise limits.
- Limits are taken from other products or processes without validation.
- “Manufacturer’s settings” for equipment are used as limits without confirming that they achieve hazard control.
Example: A sous-vide operation used “pouch fully inflated” as a cooking endpoint instead of validated temperature/time parameters - breaching both Codex and national food safety authority requirements.
4. Monitoring Records That Don’t Match Practice
Principle 4 failures are common because monitoring is treated as a form-filling exercise.
- Logs are completed in advance “to save time.”
- Identical readings appear for weeks, indicating no actual measurement.
- The monitoring frequency stated in the HACCP plan does not match what is done in practice.
Example: An audit revealed that a pasteurizer operator filled in daily logs from memory at the end of the week, with no actual temperature verification during production.
5. Lack of Real Verification
Principle 6 failures happen when verification is reduced to a paperwork review.
- Calibration is overdue or undocumented - especially for thermometers and scales used at CCPs.
- Internal HACCP audits skip observation of CCP monitoring entirely.
- Microbiological results are filed but never compared to process controls.
Example: A salad plant was cited because its internal audit “verified” that metal detector checks were recorded, but did not verify the detector’s function with test pieces during the audit.
6. Ineffective Corrective Actions and Follow-Up
Principle 5 requires immediate correction and prevention of recurrence. Failures occur when:
- The same deviation appears repeatedly in audit history.
- Corrective actions are vague (“operator retrained”) with no root cause analysis.
- No check is made to confirm the action worked.
Example: A ready meal site repeatedly recorded blast chiller temperatures above the critical limit but took no equipment maintenance action - only retraining staff - leading to repeated non-conformances.
7. Ignoring Prerequisite Failures That Undermine HACCP
Even with a solid hazard plan, GMP lapses can lead to HACCP failure.
- Pest control inspections were missed for several months.
- Cleaning schedules marked “done” without verification swabs.
- Allergen changeovers were not checked with protein testing despite being in the plan.
Example: A confectionery plant with a good HACCP plan failed an audit because protein swab results for allergen changeover were not reviewed - allowing possible cross-contact to go unnoticed.
Key takeaway: Many HACCP audit failures are traceable to a mismatch between what the plan says and what actually happens. Auditors look for consistency between documented hazards, CCPs, limits, and on-floor practices - and any break in that chain can result in a failed audit.
How AI Can Simplify HACCP Audit Preparation
After seeing the range of issues that can derail a HACCP audit, it’s clear that the most common failures stem from missed details, outdated documents, and gaps in verification. These are exactly the kinds of problems that technology - especially AI - can prevent. By integrating AI-driven tools into your food safety management process, you can automate repetitive compliance checks, maintain up-to-date documentation, and flag risks before they appear in a HACCP internal audit or third-party assessment.
IONI’s HACCP Builder, which uses AI to guide teams through every stage of HACCP plan creation, maintenance, and HACCP auditing readiness, can help businesses.
Here’s how AI simplifies preparation:
1. Automating Pre-Audit Checks
Instead of manually reviewing every record before an audit, AI can scan monitoring logs, supplier records, calibration certificates, and corrective action reports to identify gaps instantly.
2. Generating Complete and Compliant Plans
AI can build and update HACCP plans based on your current product range, processes, and hazard profile. By referencing Codex guidelines and regulatory requirements, it ensures every hazard analysis, CCP, and verification step is fully documented and audit-ready.
3. Continuous Audit Readiness
Traditional HACCP documentation often falls out of date between audits. AI tools maintain live, version-controlled records that update automatically when processes change. This means your documentation mirrors your actual operations year-round - not just during audit season.
4. Smart Alerts and Corrective Action Tracking
AI can send alerts when a CCP monitoring log is missed, a calibration is overdue, or a supplier certificate expires. Corrective actions can be logged, assigned, and tracked to closure, ensuring every issue has a documented resolution.
5. Data-Driven Insights for Continuous Improvement
Beyond basic compliance, AI can analyze audit data trends - identifying recurring issues, seasonal risks, or training gaps.
Watch this short video to see how IONI guides you through building a complete HACCP plan, from identifying hazards to setting critical control points. See the process in action and learn how easy compliance management can be!
1. Upload Your Foundation Documents
Begin by uploading your existing SOPs, process descriptions, flow diagrams, or any related documents.
IONI’s AI scans and extracts essential information - product descriptions, process steps, control measures - creating a base for your HACCP plan.
2. AI Validates Against Standards
Once documents are uploaded, IONI checks them against local and global food safety frameworks - Codex, EU, FDA, GFSI, or regional requirements. It flags misalignments or missing regulatory components early in the process.
3. Auto-Fill & Gap Completion
IONI “reads” your content, identifies gaps - such as missing hazards, absent CCPs, or unaddressed steps - and then automatically fills them in. It might suggest adding a CCP, a corrective action, or an SOP for a process step that wasn’t documented.
4. Generate SOPs Automatically
Seeing your process in visual form is powerful. IONI generates SOPs if they’re missing. These are editable, customizable, and exportable with a click.
5. Review & Customize
Every suggestion remains under your control. You can manually edit hazards, CCPs, critical limits, or any part of the plan to match your operations.
6. Real-Time Validation & Progress Tracking
IONI continuously evaluates your plan’s completeness. Missing elements are flagged, such as lacking SOPs or incomplete hazard analysis. A progress interface helps you track your path toward a fully audit-ready HACCP plan.
7. Finalize and Download Audit-Ready Documents
When everything is complete - from hazard analysis and CCPs to SOPs - you generate a polished, structured HACCP plan in PDF format. Ready to present during an internal or third-party HACCP audit.

Start transforming your HACCP management today - review your current plan, schedule your next internal audit, and explore AI-powered solutions like IONI HACCP Builder to stay audit-ready year-round.
Why This Matters for HACCP Auditing
- Speed without compromise: A plan that once took weeks to draft can now be generated in under an hour - yet remains customizable and accurate.
- Gap-proof planning: IONI actively prevents the common audit failures we previously reviewed - like missing CCPs or undocumented SOPs.
- Consistency and version control: Changes are tracked in digital form, supporting better document control and audit readiness.
- Audit alignment by design: Each element produced in IONI maps back to key HACCP principles - hazard analysis, CCPs, corrective actions, verification, and recordkeeping - creating traceable compliance.
In Summary: Your HACCP Plan, Smarter
Using IONI’s AI-powered HACCP Builder aligns the ease of automation with the precision of compliance. You start by uploading your existing documentation. AI validates it, supplements it where needed, generates required visuals and procedures, and guides you to completion - all with an audit-ready outcome tailored to your operations. For any business serious about food safety, HACCP internal audit preparation becomes a strategic advantage.
Watch How IONI Works
Watch this short video to see how IONI guides you through building a complete HACCP plan, from identifying hazards to setting critical control points. See the process in action and learn how easy compliance management can be!
Post-Audit: What to Do After the Inspection
Having used AI tools like IONI to prepare a comprehensive HACCP plan and approach your HACCP audit with confidence, the real test begins once the inspection ends. Whether it’s an internal review or a third-party certification, what you do immediately after the audit often determines how effectively you protect food safety and maintain compliance.
This stage is about responding to findings, embedding improvements into your system, and ensuring that your HACCP internal audit cycle continuously strengthens your operations.
1. Review Findings Objectively
Start by carefully reading the audit report. Distinguish between:
- Critical findings – immediate food safety hazards that require urgent action.
- Major findings – systemic issues that could lead to hazards if not addressed.
- Minor findings – isolated lapses that still need correction.
Refer back to the HACCP Principles & Application Guidelines from the FDA, especially the requirement that “a criterion is a requirement on which a judgment or decision can be based” - this ensures your corrective measures directly address the criteria your plan is built upon.
2. Prioritize and Assign Corrective Actions
For each non-conformity:
- Define the specific issue and the HACCP criterion it violates.
- Assign responsibility to the right person or team.
- Set realistic but prompt deadlines.
- Ensure all actions are documented for the next HACCP auditing cycle.
3. Implement Root Cause Analysis
Instead of patching symptoms, find out why the issue occurred.
- Was it due to unclear procedures?
- Gaps in training?
- Equipment malfunction?
Once the root cause is identified, adapt processes, training, or equipment maintenance schedules to prevent recurrence.
4. Update Your HACCP Plan
Changes made after an audit should be formally integrated into your plan:
- Revise hazard analysis tables.
- Adjust CCP definitions or critical limits if needed.
- Add or update verification and monitoring records.
- Ensure process flow diagrams reflect current reality.
5. Communicate and Train
Staff should understand not only what changed but why. Short, targeted training sessions help ensure that corrective measures stick and are applied consistently across shifts.
6. Close the Loop
Finally, schedule a follow-up HACCP internal audit or verification check to confirm that corrective actions are effective. Keep evidence - such as updated records, training logs, and verification results - ready for the next inspection.
Audit-Ready All Year: Tips for Ongoing Compliance
After responding to findings and updating your HACCP plan post-inspection, the next step is to make continuous compliance a standard part of your operations. Preparing for a HACCP audit must be embedded in everyday practices. By maintaining ongoing oversight, scheduling regular reviews, and leveraging digital tools, your team can stay consistently audit-ready.
1. Schedule Regular Internal Audits
Internal audits are the backbone of continuous compliance:
- Establish a recurring audit calendar covering all products, processes, and sites.
- Rotate auditors to maintain independence from the processes being reviewed.
- Use checklists aligned with regulatory requirements to catch gaps early.
Regular HACCP internal audits help detect deviations before they escalate into major findings and allow for timely corrective actions.
2. Maintain Comprehensive, Updated Documentation
- Keep all hazard analyses, CCP monitoring logs, SOPs, and verification records current.
- Ensure version control so every change is traceable.
- Document training sessions, calibration records, and corrective actions.
Consistent recordkeeping satisfies auditors and provides a reliable reference during day-to-day operations, reducing errors and confusion.
3. Integrate Digital Tools for Continuous Oversight
AI-driven and digital compliance platforms, such as IONI, can automate many aspects of HACCP auditing:
- Real-time monitoring of CCPs and process deviations.
- Automatic alerts for overdue verifications or missing records.
- Digital storage of SOPs, hazard analyses, and audit reports for easy retrieval.
- Progress tracking and analytics for management reviews.
These tools minimize manual workload, reduce human error, and keep your system aligned with regulatory expectations year-round.
4. Implement a Culture of Continuous Improvement
- Treat internal audits as learning opportunities rather than just compliance exercises.
- Use findings to refine procedures, update training, and improve monitoring methods.
- Encourage staff engagement in reporting potential risks or inefficiencies.
By embedding continuous improvement into daily operations, your team ensures that each HACCP internal audit strengthens the workflow.
5. Review and Adapt to Regulatory Changes
Stay updated with local, national, and international regulations affecting your products and processes. Even minor changes in permissible limits, labeling requirements, or process standards can impact HACCP audit readiness. Digital systems can help track these changes and integrate them into your HACCP plan automatically.
Being audit-ready all year means shifting from reactive compliance to proactive food safety management. Scheduling regular internal audits, maintaining meticulous records, leveraging digital tools, and fostering a culture of continuous improvement ensure that your HACCP system remains robust, traceable, and always prepared for inspection.
Conclusion
Preparing for a HACCP audit is a strategic investment in food safety, operational efficiency, and regulatory confidence. From building a robust HACCP plan and conducting structured HACCP internal audits to leveraging AI tools for continuous readiness, each step strengthens your system and reduces the risk of non-conformities.
Key takeaways include:
- Proactive preparation ensures that your HACCP documentation, CCP monitoring, and verification activities are complete and traceable at all times.
- Internal auditing is essential for uncovering hidden gaps, validating processes, and reinforcing staff understanding of hazards and control measures.
- Digital solutions and AI streamline pre-audit checks, automate recordkeeping, and provide actionable insights, helping teams remain audit-ready year-round.
- Continuous improvement transforms findings from past audits into opportunities for stronger controls, better training, and more resilient operations.
By embedding these practices into everyday operations, businesses build a culture of compliance, quality, and accountability. Effective HACCP auditing becomes less about responding to deficiencies and more about sustaining a system that consistently delivers safe, high-quality products.
Ultimately, a well-executed HACCP plan, reinforced by diligent HACCP internal audits and modern tools, turns regulatory compliance into a competitive advantage and ensures your operations are always prepared for any inspection.


.png)
.png)





