.png)
The food industry in 2026 is navigating a level of regulatory volatility and supply chain complexity that did not exist even five years ago. Ingredient portfolios are expanding while contaminant thresholds are tightening. Global sourcing has become more fragmented, and regulatory agencies are using increasingly advanced analytical tools to detect risk patterns across supply chains.
During the second half of 2025, food recall volumes increased significantly in the United States. Industry reporting documented dramatic growth in both recall events and total units affected in Q3 and Q4 compared to earlier quarters.
At the same time, regulatory agencies intensified chemical reassessment initiatives. The FDA Human Foods Program outlined expanded priorities for contaminant monitoring and post market chemical evaluations in 2026.
In Europe, EFSA recommendations regarding toxin limits following infant nutrition recalls reinforced how rapidly Food Ingredient Regulatory thresholds can evolve
These developments illustrate the structural challenge facing ingredient compliance management. Documentation alone is insufficient. Systems must interpret, connect, and validate ingredient level data continuously.
What Food Ingredient Compliance Really Means Today
Food Ingredient Compliance in 2026 is no longer about maintaining a complete set of documents. It is about proving, in real time, that every ingredient in your portfolio meets evolving safety, labeling, and regulatory expectations across multiple jurisdictions.

As Food Ingredient Regulatory scrutiny intensifies and supply chains grow more complex, compliance has shifted from static verification to continuous, data-driven validation.
Food Ingredient Compliance
Food ingredient compliance means traceable proof of safety, origin, chemical integrity, allergen accuracy, and regulatory alignment across multiple jurisdictions simultaneously. It requires that every ingredient entering a facility can be verified against internal specifications and evolving regulatory thresholds in real time.
Historically, Food Ingredient Compliance focused on approval lists and supplier qualification. Today it includes heavy metal validation under initiatives such as the FDA Closer to Zero program.
It includes ongoing reassessment of chemical safety profiles as agencies update toxicological evaluations and much effort on traceability issues. It includes rapid responsiveness to labeling enforcement tied to origin claims, allergen disclosure accuracy, and processing aid classification.
Food Ingredient Compliance is therefore no longer static. It is conditional on regulatory interpretation that may change quarterly.
Food Ingredient Regulatory
The Food Ingredient Regulatory environment has entered a new era of digital oversight. Regulatory agencies increasingly leverage analytics and risk modeling in enforcement prioritization. The FDA has publicly referenced modernization efforts that include advanced analytical frameworks within the Human Foods Program.
This means regulators themselves are operating with data driven systems. Manufacturers that rely on manual document review are at structural disadvantage.
Food Ingredient Regulatory change now propagates rapidly across markets. A contaminant threshold update in one jurisdiction can influence retailer requirements globally. Ingredient compliance management must therefore track not only domestic regulations but cross border implications.
Ingredient compliance is increasingly intertwined with financial performance.
Why Traditional Ingredient Compliance Management Breaks at Scale
Food Ingredient Compliance is no longer about document control. It is about dynamic risk interpretation.
Artificial intelligence is becoming the enabling infrastructure that allows ingredient compliance to move from reactive verification to proactive intelligence.
Ingredient Compliance Management
Traditional ingredient compliance management was built around centralized document storage and periodic review cycles. Specifications were stored. Certificates of Analysis were archived. Regulatory updates were manually interpreted.
At a limited scale this model functioned adequately. At enterprise scale it collapses.
Consider a manufacturer managing 800 ingredients across 400 SKUs with multiple co packers. Each ingredient may carry several supplier versions, regional regulatory declarations, allergen certifications, sustainability disclosures, and contaminant test reports.
The result is thousands of active documents under management at any given time.
In late 2025, recall analysis continued to show allergen mislabeling and documentation inconsistency as major drivers of incidents.

Manual review cannot reliably detect drift across such volume. If one supplier modifies a heavy metal declaration, if another changes a specification tolerance, if an R and D team reformulates a stabilizer blend, the ripple effects may not be visible immediately.
Ingredient compliance management becomes reactive. Compliance debt accumulates. Audit preparation becomes stressful rather than systematic.
The systemic limitations of document heavy compliance processes are explored in Automating Documentation for GFSI, SQF, BRC and FSMA.
Without structured interpretation, ingredient compliance is always lagging behind operational reality.
From Documents to Data: The Shift in Ingredient Compliance
The transformation underway in ingredient compliance management is foundational. It is the transition from document centric thinking to data centric thinking.
A PDF specification is static. Structured data extracted from that specification is actionable.
When COAs are parsed into analyte fields, they can be compared automatically against internal limits. When allergen declarations are standardized into structured taxonomies, label validation becomes systematic. When contaminant values are stored as comparable parameters, trend analysis becomes possible.
This shift proved critical during contaminant related recalls in late 2025 and early 2026. Companies with structured ingredient data were able to isolate affected batches quickly. Those reliant on manual search faced delays.
Ingredient compliance becomes intelligence when data is structured and connected.
How AI Interprets Food Ingredient Regulatory Requirements
This digital transformation moves compliance from a reactive, manual bottleneck to a proactive decision-support system. AI-driven platforms automatically perform gap analyses, flagging non-compliant SKUs and predicting future shifts in "Clean Label" standards based on emerging scientific literature.
By filtering out regulatory "noise," AI allows compliance teams to focus on high-level risk management and cross-border strategy, ensuring that formulation changes are both legally sound and mathematically precise before a product ever hits the production line.
Food Ingredient Regulatory
Regulatory language is complex and frequently updated. AI systems trained on Food Ingredient Regulatory text use natural language processing to analyze new guidance documents, enforcement communications, and toxicological updates.
When EFSA revises acceptable daily intake thresholds or when the FDA updates contaminant action levels, AI systems can evaluate which ingredients and SKUs are impacted.
Reuters reporting in early 2026 highlighted precautionary recommendations affecting infant formula categories following toxin evaluations.
Ingredient compliance must absorb such updates immediately.
Ingredient Compliance
AI driven interpretation ensures ingredient compliance is maintained dynamically. Instead of waiting for manual review cycles, systems flag regulatory mismatches instantly. If an ingredient exceeds a revised threshold in a particular jurisdiction, exposure is identified across portfolios.
Regulatory agility is no longer optional. It is a prerequisite for operational continuity. AI is reshaping regulatory interpretation across the food industry, especially when we talk about needed food audit software that is needed for reliable ingredient compliance.
Ingredient compliance management powered by AI becomes anticipatory rather than reactive.
Automating Specs, COAs and Supplier Documents With AI
The operational heart of ingredient compliance management lies in supplier document exchange and HACCP software.
Computer vision and AI driven extraction technologies read COAs, normalize units, compare results to specifications, and flag deviations instantly. Automated validation eliminates arithmetic errors and ensures consistent interpretation across global suppliers.
This capability is foundational for scalable Food Ingredient Compliance.
Ingredient compliance management becomes sustainable when automation handles volume and humans focus on interpretation.
AI Powered Ingredient Traceability Across Suppliers and Co Packagers
Beyond mere record-keeping, these systems employ predictive analytics and anomaly detection to safeguard supply chain integrity. AI-powered platforms can automatically flag unauthorized ingredient substitutions at a co-packer's facility or detect subtle temperature deviations during transit that might compromise safety.
By converting static documentation into a dynamic, searchable knowledge graph, AI enables food brands to verify mass balance, manage allergens across multiple sites, and uphold "Clean Label" promises with absolute transparency, regardless of how many hands the product passes through before reaching the consumer.
Ingredient Traceability Software
Ingredient traceability software has moved from being a recall support utility to becoming a core compliance infrastructure layer.
In 2026, traceability is no longer measured only by the ability to retrieve shipping records. It is measured by the ability to reconstruct ingredient lineage at batch level, across supplier changes, co packer transitions, and reformulation cycles.
Although FSMA Rule 204 enforcement deadlines extend further, retailer requirements have accelerated implementation expectations. Many major buyers now require digital traceability readiness as a contractual baseline rather than a future target. Industry summaries throughout 2025 repeatedly highlighted that traceability preparedness is no longer optional for suppliers entering large retail ecosystems.
Ingredient traceability software powered by AI links specification data, lot level COAs, production batches, and finished goods into a coherent chain. If a contaminant alert is issued for a specific raw material batch, affected SKUs can be isolated immediately.
The difference between manual traceability and AI powered ingredient traceability software is speed and precision. Manual systems often require hours or days to reconstruct affected product scope. AI systems can perform impact analysis in seconds because relationships are already mapped.
The strategic importance of digital recall readiness is explored in Food Safety Software for Food Recall Management Full Guide 2026.
Ingredient traceability software like IONI strengthens Food Ingredient Compliance because it connects regulatory defensibility with operational visibility.
Ready to try? Feel free to book a demo with us and see how IONI may help you.
Detecting Ingredient Compliance Risks Before Audits and Recalls
The most valuable application of AI in ingredient compliance management is early risk detection.
Many compliance failures are not sudden. They are gradual. Contaminant levels may trend upward within acceptable ranges. Allergen statements may shift subtly between supplier lots. Moisture or microbiological parameters may drift over time.
Human reviewers often evaluate data in isolation. AI evaluates patterns.
In the second half of 2025, several analyses noted an increase in recalls triggered by undeclared allergens and documentation inconsistencies. Pattern recognition could have identified early warning signals in many of those cases.
Food fraud remains another persistent vulnerability. The Food and Agriculture Organization continues to emphasize the economic and safety impacts of adulteration and misrepresentation within global supply chains.
AI driven ingredient compliance management identifies anomalies across supplier history. If a supplier’s heavy metal values trend upward across multiple shipments, even while remaining technically compliant, the system flags risk accumulation.
This transforms ingredient compliance from retrospective verification into predictive oversight. Continuous monitoring creates a state of ongoing audit readiness rather than episodic audit preparation.
What to Look for in AI Driven Ingredient Compliance Software
Choosing AI driven ingredient compliance management software in 2026 is not about finding a faster document storage system. It is about determining whether your organization can sustain Food Ingredient Compliance under constant regulatory pressure, supplier variability, and portfolio expansion.
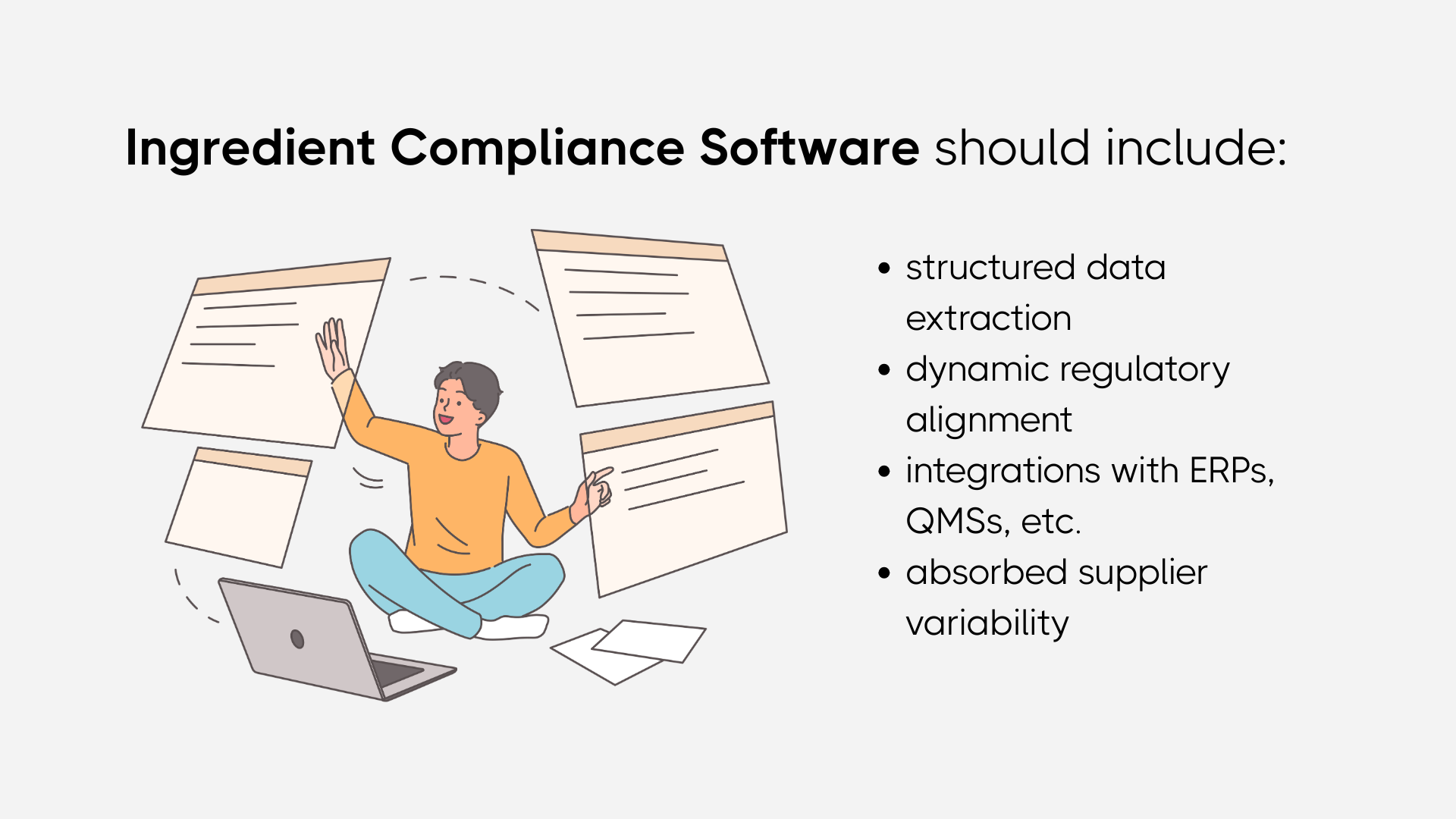
The first capability that truly matters is structured data extraction. If the system cannot reliably read specifications, Certificates of Analysis, and allergen declarations and convert them into comparable, normalized data, then ingredient compliance remains manual at its core. AI must understand units of measurement, tolerances, contaminant limits, and specification ranges. Without this, the platform is simply a digital filing cabinet.
The second requirement is dynamic regulatory alignment. Food Ingredient Regulatory thresholds change. Chemical reassessments happen. Contaminant guidance evolves. The software must be capable of evaluating regulatory impact across the portfolio without requiring weeks of manual cross checking. Ingredient compliance management should not rely on someone remembering to compare new limits against old specifications. The system should surface exposure automatically.
Third, supplier variability must be absorbed, not amplified. Every supplier formats documentation differently. Some use ppm, others mg per kg. Some provide target ranges, others provide single value results. AI driven ingredient compliance management must normalize these differences instantly. If your team is still manually reinterpreting supplier COAs, the system is not doing its job.
Integration is another non-negotiable factor. Ingredient compliance does not operate in isolation. It intersects with formulation systems, ERP platforms, quality management workflows, and ingredient traceability software. A system that cannot connect lot level traceability with structured compliance data will create blind spots during audits and recalls.
Explainability is equally critical. When the system flags an ingredient as non compliant, it must clearly show why. Which analyte triggered the alert. Which internal specification or regulatory threshold was exceeded. Which shipment or lot was affected. In regulated environments, compliance decisions must be defensible.
Finally, the system should provide ROI. Ingredient compliance management should not only detect failures but be effective for the business. Otherwise, it does not make any sense for business owners to invest in it.
Effective AI driven ingredient compliance software converts documentation into structured intelligence, interprets Food Ingredient Regulatory change continuously, integrates with ingredient traceability software, and provides transparent reasoning. Anything less is incremental improvement. What manufacturers need now is structural transformation.
IONI
The evolution of ingredient compliance management requires more than incremental automation. It requires a structural shift in how ingredient data, supplier documentation, and Food Ingredient Regulatory requirements are interpreted and connected.
IONI was built specifically around that structural shift.
Rather than functioning as a document repository, IONI operates as an intelligence layer across Food Ingredient Compliance workflows for food manufacturing. Its architecture is designed to ingest unstructured supplier documentation, convert it into structured ingredient level data, and continuously validate that data against internal specifications and regulatory thresholds.
At its core, IONI treats ingredient compliance management as a connected system rather than a collection of files.
When a new specification is uploaded, the platform parses it into analyte level parameters. When a Certificate of Analysis arrives, IONI extracts measurement values, normalizes units, and compares results directly against defined limits. This eliminates the arithmetic errors and manual review delays that often introduce compliance gaps.
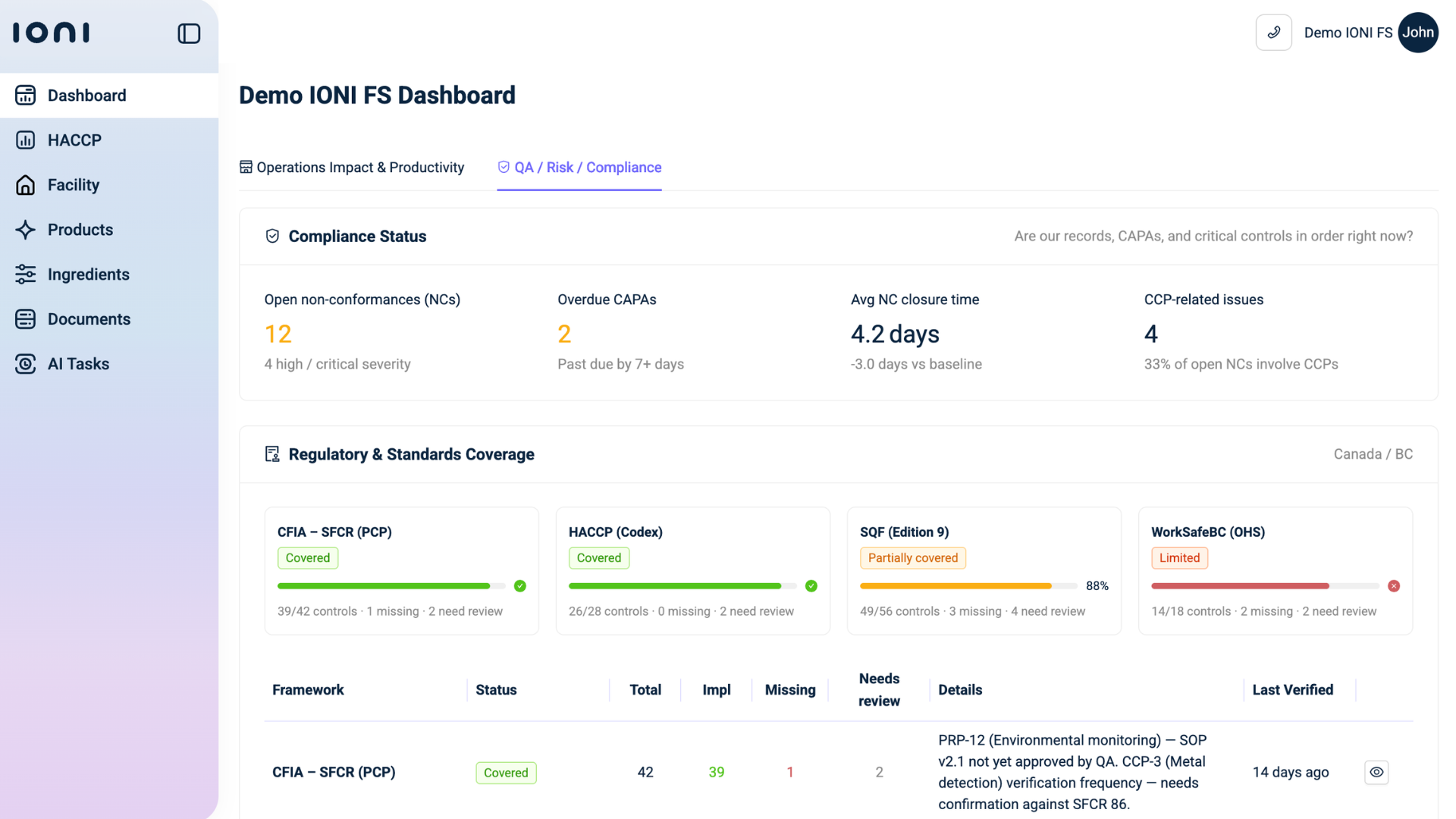
More importantly, IONI does not treat each document in isolation. It connects specification data to formulation data, supplier profiles, and product portfolios. If a supplier modifies a contaminant declaration, the impact is assessed across all SKUs that use that ingredient. If a regulatory threshold changes under Food Ingredient Regulatory updates, exposure is evaluated across active formulations.
This interconnected logic transforms Food Ingredient Compliance from reactive document validation into continuous portfolio level risk assessment.
IONI’s AI agents are trained specifically on regulatory language and supplier documentation structures. They are capable of distinguishing between mandatory contaminant limits and advisory guidance, between maximum permitted levels and typical values, between specification ranges and target values. This nuance is essential for maintaining ingredient compliance under shifting regulatory interpretation.
In practical terms, this means ingredient compliance management no longer depends on individuals remembering to cross check updates. The system performs that validation continuously.
IONI also integrates ingredient traceability software functionality at lot level. Every incoming batch can be linked to structured specification data, supplier performance history, and finished goods output. If a contaminant alert is issued upstream, the system can instantly identify which SKUs are affected and which markets are exposed.
This lot level intelligence reduces both recall risk and audit vulnerability. It ensures that ingredient traceability software is not a standalone module but embedded into the broader compliance architecture.
Another defining characteristic of IONI is explainability. In regulated environments, AI outputs must be defensible. Every flagged deviation, every regulatory mismatch, every compliance alert is accompanied by structured reasoning. The platform shows which parameter triggered the issue, which threshold was applied, and which regulation or internal limit was referenced.
This transparency is critical for regulatory inspections and third party audits. Food Ingredient Compliance decisions must be traceable, not opaque.
IONI also adapts to internal standards rather than enforcing generic templates. Ingredient compliance management differs across companies based on risk tolerance, product category, and market footprint. The platform learns internal specification structures and applies them consistently across supplier documentation.
As portfolios grow, IONI scales horizontally. It can manage hundreds or thousands of ingredients without degrading validation speed. This scalability is essential because ingredient complexity does not plateau. It increases as companies expand into new categories and regions.
From a governance perspective, IONI centralizes ingredient compliance management into a single structured environment. Regulatory updates, supplier performance trends, contaminant drift, allergen consistency, and traceability data are visible within one system rather than scattered across spreadsheets and shared drives.
This centralization strengthens audit readiness. During inspections, evidence can be retrieved instantly. Specification history, COA comparisons, regulatory interpretations, and lot traceability chains are structured and accessible.
In 2026, regulators themselves are leveraging analytics and predictive models in oversight prioritization. IONI enables manufacturers to operate at the same level of sophistication. Ingredient compliance management becomes analytical rather than clerical.
The practical outcome is measurable. Manual document review time decreases significantly. Regulatory response speed increases. Portfolio level risk visibility improves. Ingredient compliance becomes sustainable even as regulatory complexity grows.
IONI does not replace regulatory professionals. It amplifies their capability. Instead of spending hours reviewing PDFs, compliance teams focus on interpretation, supplier engagement, and strategic risk mitigation.
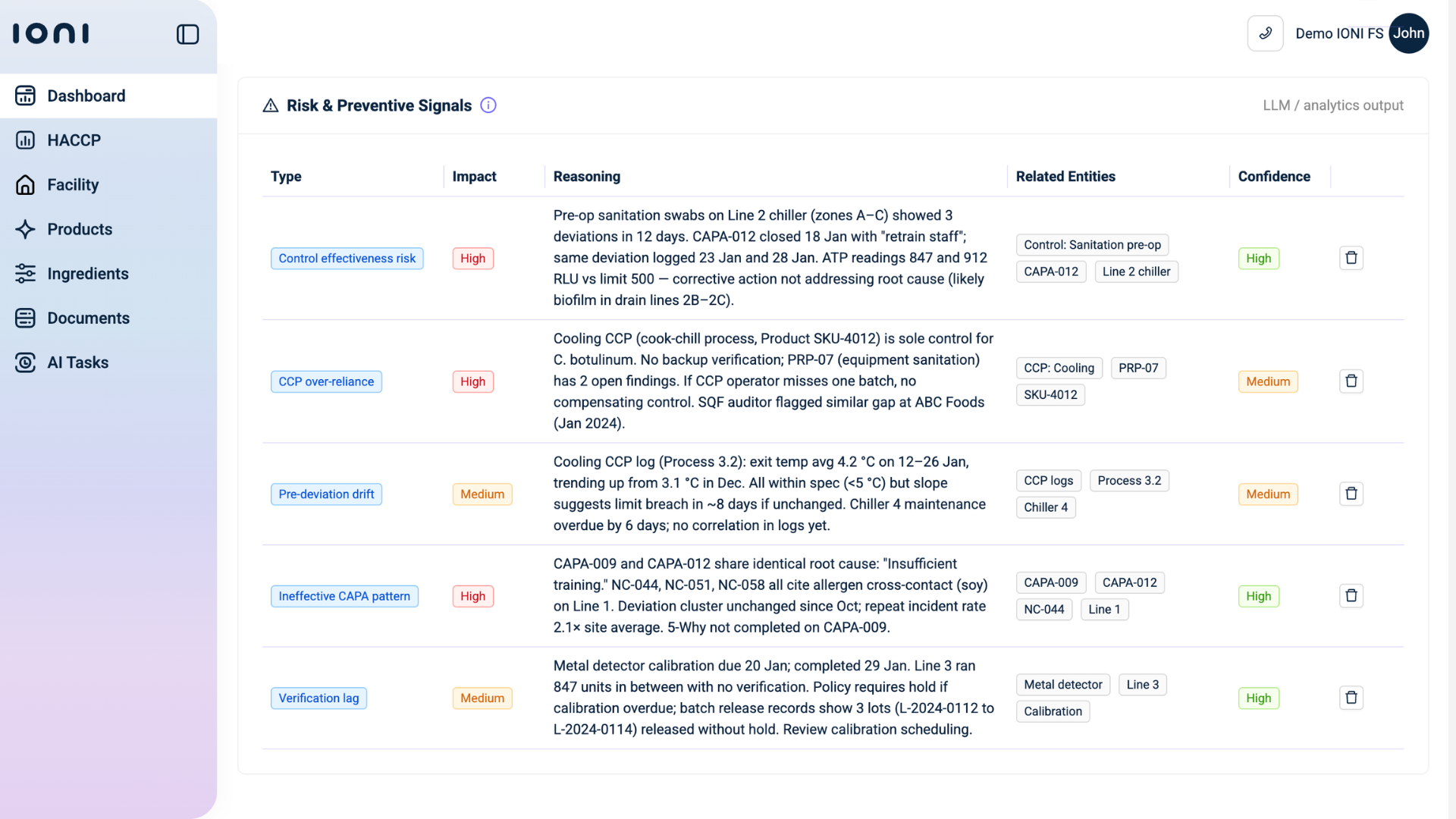
In a regulatory climate defined by continuous change, this shift is not a luxury. It is infrastructure.
Ready to try? Feel free to book a demo with us and see how IONI may help you.
Why Ingredient Compliance Management Is Becoming a Competitive Advantage
Here is the revised section with clear, strong advantage bullets added in a strategic way. The tone remains professional and not overly promotional.
Why Ingredient Compliance Management Is Becoming a Competitive Advantage
Ingredient compliance management is no longer just about avoiding recalls or passing audits. In 2026, it directly impacts speed to market, retailer trust, and operational resilience.
Retailers expect immediate ingredient transparency. Regulators expect structured, defensible evidence. Consumers expect clarity around sourcing and safety. Companies that can demonstrate Food Ingredient Compliance instantly move faster than competitors still dependent on manual validation and fragmented documentation.

This is where IONI creates measurable strategic advantage.
IONI reduces the time required to validate new ingredients from days to minutes. When a new stabilizer, protein source, or additive is introduced, the platform evaluates contaminant thresholds, allergen exposure, and Food Ingredient Regulatory alignment automatically. Reformulation cycles accelerate without increasing risk.
IONI also strengthens supplier oversight. Because the platform structures historical COA data and performance patterns, ingredient compliance management becomes predictive rather than reactive. Procurement and quality teams gain portfolio level visibility that was previously impossible.
The competitive advantages become tangible:
- Faster product launches because regulatory validation happens in parallel with formulation
- Reduced recall exposure through early contaminant drift detection
- Stronger supplier negotiations supported by structured performance data
- Immediate audit readiness with structured, explainable compliance evidence
- Confident market expansion through automated Food Ingredient Regulatory impact analysis
- Precision recall containment enabled by integrated ingredient traceability software
IONI transforms ingredient compliance from documentation control into portfolio intelligence. Instead of reacting to regulatory changes, the system continuously evaluates exposure. Instead of spending hours reviewing PDFs, teams focus on risk interpretation and supplier strategy.
In a market where transparency determines retailer partnerships and regulatory agility determines growth, ingredient compliance management becomes a competitive differentiator. With IONI, it becomes infrastructure for scale rather than overhead to manage.
Ready to try? Feel free to book a demo with us and see how IONI may help you.
Conclusion
Ingredient compliance management is undergoing a structural transformation.
Food Ingredient Compliance today requires continuous validation across chemical safety, allergen accuracy, origin transparency, and regulatory alignment. Food Ingredient Regulatory expectations evolve faster than manual systems can absorb. Ingredient traceability software has become foundational infrastructure. AI is emerging as the mechanism that makes continuous compliance feasible.
The increase in recalls during the second half of 2025, the tightening of contaminant thresholds in early 2026, and the modernization of regulatory oversight frameworks all signal the same direction. Ingredient compliance cannot rely on documents alone.
AI driven ingredient compliance management transforms static records into structured intelligence. It identifies drift before thresholds are exceeded. It interprets regulatory updates automatically. It connects supplier documentation to portfolio level risk analysis.
The organizations that adopt structured, AI powered ingredient compliance systems will experience fewer recall events, faster audit cycles, and stronger retailer trust. Those that rely solely on manual workflows will find themselves increasingly reactive in a regulatory environment that demands precision and speed.
Ingredient compliance is no longer about passing inspections. It is about sustaining defensibility every day.


.png)






